Is early exposure to acetaminophen and NSAIDs safe for the male reproductive system
While pregnant women often self-medicate with acetaminophen (paracetamol/APAP) or NSAIDs such as ibuprofe...
View More
Chicago, USA
Dear Esteemed Colleagues,
We are delighted to extend a heartfelt welcome to the forthcoming "6th Global Summit on Advances in Medicinal Chemistry & Pharmacology," scheduled to take place in the dynamic city of Amsterdam, Netherlands on April 03-04, 2025.
This year's conference assures an exceptional experience featuring engaging sessions, lectures, and presentations by distinguished experts and editors of esteemed journals. Featuring an extensive and diverse agenda meticulously crafted by a renowned international faculty, this year's conference will center around the compelling theme, "Innovations Shaping the Future: Transformative Technologies in Medicinal Chemistry & Pharmacology."
Our array of speakers and panelists, representing diverse sectors such as academic institutions, healthcare institutes, pharmaceuticals, biotech, CROs, supply chain, logistics, and academic scholars, is poised to deliver valuable insights and actionable tools to brainstorm new ideas and discover new skills.
This event serves as an outstanding platform for professionals to exchange research findings and stay abreast of recent innovations from colleagues worldwide. We have full confidence that all attendees, encompassing students, experts, and policy-makers, will derive substantial benefits from their participation in Adv. Med Chem 2025.
We look forward to a highly informative and stimulating meeting, with critical deliberations and the opportunity to meet new colleagues working in this important field.
With best regards,
Adv. Med Chem 2025
Organizing Committee
Peers Alley Media, Canada

Harvard Medical School,
USA
Cleveland Diagnostics,
USA
Antares Health Products Inc., East Tennessee State University,
USA
University of Illinois,
USA
Genentech,
USA
Theoretical Cognitive Neurobiology Practitioner at Sydney,
Australia
Jagiellonian University,
Poland
Mayo Clinic,
USA









Drugs and Drug Targets

Drugs are substances that are used to treat, cure, or prevent diseases or medical conditions. They can be synthetic or naturally occurring and can come in different forms such as pills, capsules, injections, patches, and liquids.
Drug targets are specific molecules or structures in the body that drugs interact with to produce their therapeutic effects. These targets can be proteins, enzymes, receptors, ion channels, or other cellular components. Drugs can either activate or inhibit these targets, leading to various biological responses.
The identification and validation of drug targets are crucial for the development of new drugs. Researchers typically search for targets that are involved in the disease process or the biological pathways that are disrupted in the disease. Once a potential target is identified, researchers will design and test drugs that can interact with the target in a specific way to produce a therapeutic effect.
The drug discovery and development process involves several stages, including target identification, drug design, preclinical testing, clinical trials, and regulatory approval. It can take many years and billions of dollars to bring a new drug to market.
Overall, drugs and drug targets play a critical role in modern medicine and are essential tools for treating and managing diseases.
Global Market:
The global drugs and drug targets market is a complex and constantly evolving industry that includes a wide range of products, technologies, and services aimed at developing, manufacturing, and distributing drugs for various therapeutic purposes. The market is driven by various factors, including increasing healthcare expenditure, growing demand for personalized medicine, and rising prevalence of chronic diseases such as cancer, diabetes, and cardiovascular diseases.
According to a report by Grand View Research, Inc., the global drugs and drug targets market size was valued at USD 120.5 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 8.8% from 2021 to 2028. The increasing investment in research and development activities, the growing adoption of targeted therapies, and the emergence of novel drug targets are some of the major factors driving market growth.
The market is segmented based on drug type, therapeutic area, technology, and region. Based on drug type, the market is segmented into small molecules, biologics, and vaccines. Based on therapeutic area, the market is segmented into oncology, cardiovascular diseases, central nervous system disorders, infectious diseases, and others. Based on technology, the market is segmented into genomics, proteomics, and metabolomics.
North America dominated the global drugs and drug targets market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and increasing investment in research and development activities.
Overall, the drugs and drug targets market is expected to experience significant growth in the coming years, driven by technological advancements, increasing demand for personalized medicine, and the rising prevalence of chronic diseases.
Drug Discovery, Design and Development

Drug discovery, design, and development are complex processes that involve identifying new targets, designing and synthesizing molecules, testing their efficacy and safety, and bringing them to market. Here is a brief overview of each of these steps:
Target Identification: Researchers identify targets that are involved in the disease process or the biological pathways that are disrupted in the disease. They use various methods, such as high-throughput screening, genetic analysis, and computer modeling to identify potential targets.
Lead Discovery: Researchers use different techniques, such as virtual screening, high-throughput screening, and fragment-based drug design to identify lead compounds that can interact with the target. The lead compounds are then optimized to improve their efficacy, potency, and selectivity.
Preclinical Testing: Researchers test the lead compounds in preclinical models, such as cells, tissues, and animal models, to evaluate their safety, pharmacokinetics, and pharmacodynamics. This stage aims to identify potential toxicities, establish the optimal dose range, and confirm the mechanism of action.
Clinical Trials: The lead compound undergoes a series of clinical trials to evaluate its safety, efficacy, and optimal dosage. Clinical trials involve testing the drug in humans, and they are conducted in three phases: Phase I, Phase II, and Phase III.
Regulatory Approval: After completing clinical trials, the company submits a New Drug Application (NDA) to the regulatory authorities, such as the Food and Drug Administration (FDA), for approval. The NDA contains data from preclinical and clinical studies, manufacturing details, labeling, and proposed use.
Market Launch: If the regulatory authority approves the drug, it can be launched in the market. The company continues to monitor the safety and efficacy of the drug through post-marketing surveillance.
The drug discovery, design, and development process can take several years and require significant investment, expertise, and collaboration among researchers, clinicians, and industry partners. However, the successful development of a new drug can have a significant impact on patients' lives, and it can lead to improved treatments and better health outcomes.
Global Market:
The global drug discovery, design, and development market is a rapidly growing industry that encompasses a wide range of activities involved in the process of developing new drugs for various therapeutic areas. This market includes various stages of drug development, including target identification, lead generation, preclinical testing, clinical trials, and regulatory approval.
According to a report by Grand View Research, Inc., the global drug discovery, design, and development market size was valued at USD 133.1 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 11.2% from 2021 to 2028. The increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular diseases, along with rising demand for personalized medicine, is driving market growth.
The market is segmented based on service, product, and region. Based on service, the market is segmented into drug discovery, drug design, and drug development. Based on product, the market is segmented into software and services. The software segment is further sub-segmented into molecular modeling and simulation, and bioinformatics tools.
North America dominated the global drug discovery, design, and development market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and increasing investment in research and development activities.
The key players operating in the global drug discovery, design, and development market include Thermo Fisher Scientific, Inc., Merck & Co., Inc., Charles River Laboratories International, Inc., GVK Biosciences Private Limited, WuXi AppTec Group, and Evotec SE, among others.
Overall, the drug discovery, design, and development market is expected to experience significant growth in the coming years, driven by technological advancements, increasing demand for personalized medicine, and the rising prevalence of chronic diseases.
Pharmacokinetics and Pharmacodynamics

Pharmacokinetics and pharmacodynamics are two important concepts in pharmacology that describe how drugs interact with the body.
Pharmacokinetics refers to the study of how the body affects drugs. This includes the processes of absorption, distribution, metabolism, and elimination of drugs from the body. These processes determine the concentration of drugs in the body over time and how long they remain active.
Absorption: The process by which drugs enter the bloodstream after being administered through various routes such as oral, intravenous, or topical administration.
Distribution: The process by which drugs are transported through the bloodstream to the target tissues or organs where they exert their therapeutic effect.
Metabolism: The process by which drugs are transformed by enzymes in the liver or other tissues into inactive or active metabolites.
Elimination: The process by which drugs are eliminated from the body through urine, feces, or breath.
Pharmacodynamics refers to the study of how drugs affect the body. This includes the mechanisms of action, therapeutic effects, side effects, and toxicities of drugs.
Mechanism of Action: The specific biochemical or physiological pathways by which drugs interact with their targets to produce a therapeutic effect.
Therapeutic Effect: The desired effect of drugs in treating or preventing diseases or medical conditions.
Side Effects: The unintended effects of drugs that can occur in addition to the therapeutic effects.
Toxicity: The harmful effects of drugs on the body, which can be dose-dependent or idiosyncratic.
Pharmacokinetics and pharmacodynamics are closely interrelated and affect each other. The concentration of drugs in the body (pharmacokinetics) determines the intensity and duration of their pharmacological effects (pharmacodynamics). Understanding these concepts is essential in the development and use of safe and effective drugs.
Global Market:
The global pharmacokinetics and pharmacodynamics market is a rapidly growing industry that encompasses the study of the movement of drugs within the body (pharmacokinetics) and the effects of drugs on the body (pharmacodynamics). This market includes various services and products related to drug metabolism, drug-drug interactions, and drug toxicity.
According to a report by MarketsandMarkets, the global pharmacokinetics and pharmacodynamics market size is expected to reach USD 6.8 billion by 2025, growing at a compound annual growth rate (CAGR) of 6.6% from 2020 to 2025. The increasing prevalence of chronic diseases and the rising demand for personalized medicine are driving market growth.
The market is segmented based on application, end-user, and region. Based on application, the market is segmented into drug discovery and development, drug interactions, drug metabolism, and other applications. Based on end-user, the market is segmented into pharmaceutical and biopharmaceutical companies, contract research organizations (CROs), and other end-users.
North America dominated the global pharmacokinetics and pharmacodynamics market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and increasing investment in research and development activities.
The key players operating in the global pharmacokinetics and pharmacodynamics market include Charles River Laboratories International, Inc., Covance Inc., Eurofins Scientific, Thermo Fisher Scientific, Inc., and WuXi AppTec Group, among others.
Overall, the pharmacokinetics and pharmacodynamics market is expected to experience significant growth in the coming years, driven by technological advancements, increasing demand for personalized medicine, and the rising prevalence of chronic diseases.
Computer Aided Drug Design

Computer-aided drug design (CADD) is a process that uses computational methods and tools to discover, design, and optimize new drugs. CADD integrates various techniques from chemistry, biology, physics, and computer science to accelerate the drug discovery and development process.
CADD involves several steps, including:
Target Identification: CADD begins with the identification of a target, such as a protein, enzyme, or receptor, that is involved in the disease process. Computer models and simulations are used to predict the structure and function of the target.
Ligand Screening: Ligand screening involves the identification and screening of small molecules, called ligands, that can interact with the target. This can be done using virtual screening methods, which use computer algorithms to screen large databases of compounds and identify potential candidates for further testing.
Molecular Docking: Molecular docking is a computational method that predicts the binding orientation and affinity of a ligand to the target. This involves the simulation of the interactions between the ligand and the target to identify the most favorable binding sites and orientations.
Lead Optimization: Once potential lead compounds are identified, CADD is used to optimize their properties, such as potency, selectivity, and pharmacokinetics, to improve their efficacy and safety.
Preclinical Testing: The lead compounds are then tested in preclinical models, such as cells, tissues, and animal models, to evaluate their safety, pharmacokinetics, and pharmacodynamics.
Clinical Trials: The lead compounds undergo a series of clinical trials to evaluate their safety and efficacy in humans.
CADD can accelerate the drug discovery and development process by reducing the time and cost required for traditional experimental methods. It can also improve the success rate of drug development by identifying potential lead compounds with higher potency, selectivity, and safety. However, CADD is not a replacement for experimental methods, and it requires the integration of computational and experimental approaches to achieve optimal results.
Global Market:
The global computer-aided drug design (CADD) market is a rapidly growing industry that involves the use of computational tools and methods to design and discover new drugs. CADD plays a crucial role in drug discovery and development by enabling faster and more efficient screening of potential drug candidates.
According to a report by Grand View Research, Inc., the global CADD market size was valued at USD 1.1 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 16.2% from 2021 to 2028. The increasing demand for personalized medicine, rising prevalence of chronic diseases, and growing need for cost-effective drug discovery are some of the major factors driving market growth.
The market is segmented based on software, services, and region. Based on software, the market is segmented into molecular modeling and docking software, virtual screening software, and other software. Based on services, the market is segmented into drug discovery and development services, and other services.
North America dominated the global CADD market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and increasing investment in research and development activities.
The key players operating in the global CADD market include Schrödinger, LLC, Biovia (Dassault Systèmes), Chemical Computing Group ULC, OpenEye Scientific Software, Inc., and Simulations Plus, Inc., among others.
Overall, the CADD market is expected to experience significant growth in the coming years, driven by technological advancements, increasing demand for personalized medicine, and the rising prevalence of chronic diseases.
Drug Metabolism

Drug metabolism refers to the biochemical processes by which the body transforms drugs into metabolites that can be excreted from the body. These processes are essential for eliminating drugs from the body and preventing their accumulation, which can lead to toxicity.
Drug metabolism occurs primarily in the liver, but other organs such as the kidneys, lungs, and intestines can also play a role. There are two main phases of drug metabolism:
Phase I metabolism: This phase involves the modification of drugs by introducing or exposing functional groups, such as hydroxyl (-OH), amino (-NH2), or carboxyl (-COOH) groups, through oxidation, reduction, or hydrolysis reactions. These reactions are primarily carried out by enzymes called cytochrome P450 enzymes (CYPs) and can result in the formation of active, inactive, or toxic metabolites.
Phase II metabolism: This phase involves the conjugation of drugs or their Phase I metabolites with endogenous compounds, such as glucuronic acid, sulfate, or amino acids, to increase their solubility and facilitate their excretion from the body. These reactions are catalyzed by enzymes called transferases, and the resulting conjugates are typically inactive and easily excreted from the body.
The rate and extent of drug metabolism can vary depending on several factors, including genetics, age, sex, diet, and other drugs. Genetic variations in CYP enzymes can affect drug metabolism and lead to individual differences in drug response and toxicity.
Drug metabolism can also give rise to drug-drug interactions, where the metabolism of one drug affects the metabolism of another drug, leading to changes in drug efficacy or toxicity. For example, a drug that inhibits CYP enzymes can decrease the metabolism of another drug, leading to increased drug levels and potential toxicity.
Understanding drug metabolism is essential for the development of safe and effective drugs and for optimizing drug therapy for individual patients.
Global Market:
The global drug metabolism market is a rapidly growing industry that encompasses the study of the biochemical and physiological processes that transform drugs into metabolites within the body. This market includes various products and services related to drug metabolism, including in vitro and in vivo testing, metabolite identification, and drug-drug interaction studies.
According to a report by MarketsandMarkets, the global drug metabolism market size is expected to reach USD 4.65 billion by 2025, growing at a compound annual growth rate (CAGR) of 7.4% from 2020 to 2025. The increasing demand for personalized medicine and the rising prevalence of chronic diseases are driving market growth.
The market is segmented based on product and service, application, and region. Based on product and service, the market is segmented into instruments, software, and services. Based on application, the market is segmented into drug discovery and development, drug metabolism studies, and other applications.
North America dominated the global drug metabolism market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and increasing investment in research and development activities.
The key players operating in the global drug metabolism market include Thermo Fisher Scientific, Inc., Eurofins Scientific, Charles River Laboratories International, Inc., Cyprotex PLC, and General Electric Company, among others.
Overall, the drug metabolism market is expected to experience significant growth in the coming years, driven by technological advancements, increasing demand for personalized medicine, and the rising prevalence of chronic diseases.
Pharmaceutical Biotechnology

Pharmaceutical biotechnology is a field that applies biotechnology techniques to the development, manufacturing, and regulation of drugs. It involves the use of living systems and biological processes to create and modify drugs and biologics, such as vaccines, gene therapies, and monoclonal antibodies.
Pharmaceutical biotechnology encompasses several areas, including:
Recombinant DNA Technology: This involves the use of genetic engineering techniques to modify the DNA of cells to produce proteins with therapeutic properties. This has led to the development of biologics, such as insulin, growth hormones, and monoclonal antibodies.
Cell Culture Technology: This involves the use of cells to produce therapeutic proteins, including the use of recombinant DNA technology to modify cells to produce specific proteins.
Protein Engineering: This involves the modification of proteins to improve their efficacy, stability, and pharmacokinetics, and to reduce their immunogenicity. This can include the use of genetic engineering techniques to modify protein structures and the use of chemical modifications to alter protein properties.
Genomics and Proteomics: This involves the use of high-throughput techniques to identify new drug targets and biomarkers, and to understand the mechanisms of disease and drug action.
Regulatory Affairs: This involves the development of regulatory strategies to ensure the safety and efficacy of biologic drugs, including the evaluation of preclinical and clinical data, and the development of manufacturing and quality control procedures.
Pharmaceutical biotechnology has revolutionized the pharmaceutical industry, leading to the development of several life-saving drugs and biologics. It has also led to the development of personalized medicine, where drugs are tailored to individual patients based on their genetic makeup and disease characteristics. However, the development and manufacturing of biologics can be challenging due to the complexity of these molecules and the need for specialized manufacturing processes and quality control procedures.
Global Market:
The global pharmaceutical biotechnology market is a rapidly growing industry that involves the use of biotechnology techniques in the development and production of pharmaceuticals. This market includes various products and services related to biologics, vaccines, gene therapies, and cell therapies, among others.
According to a report by Grand View Research, Inc., the global pharmaceutical biotechnology market size was valued at USD 328.5 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 8.6% from 2021 to 2028. The increasing demand for biologics, rising prevalence of chronic diseases, and growing investments in research and development are driving market growth.
The market is segmented based on product, technology, application, and region. Based on product, the market is segmented into monoclonal antibodies, vaccines, recombinant proteins, cell therapy, gene therapy, and other products. Based on technology, the market is segmented into DNA sequencing, fermentation, cell culture, and other technologies. Based on application, the market is segmented into oncology, infectious diseases, cardiovascular diseases, and other applications.
North America dominated the global pharmaceutical biotechnology market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical and biotechnology companies, well-established healthcare infrastructure, and increasing investment in research and development activities.
The key players operating in the global pharmaceutical biotechnology market include F. Hoffmann-La Roche Ltd, Amgen Inc., Sanofi, Johnson & Johnson, and Pfizer Inc., among others.
Overall, the pharmaceutical biotechnology market is expected to experience significant growth in the coming years, driven by technological advancements, increasing demand for biologics, and the rising prevalence of chronic diseases.
Combinatorial Chemistry

Combinatorial chemistry is a method used in drug discovery to rapidly generate and screen large numbers of chemical compounds for biological activity. It involves the simultaneous synthesis of many chemical compounds, which are then screened for biological activity against a target protein or biological pathway.
Combinatorial chemistry is based on the idea that a large number of structurally diverse compounds can be synthesized using a relatively small set of building blocks and synthetic strategies. These compounds can then be screened for their ability to bind to a target protein or modulate a biological pathway. The aim is to identify lead compounds that have the desired biological activity and can be further optimized to develop drugs.
There are several methods of combinatorial chemistry, including:
Solid-phase synthesis: This involves the use of a solid support to facilitate the synthesis of large numbers of compounds. The solid support can be used to immobilize a starting material, which is then reacted with a series of reagents to generate a library of compounds.
Solution-phase synthesis: This involves the use of solution-phase reactions to generate libraries of compounds. It typically involves the use of parallel synthesis techniques to generate many compounds simultaneously.
Parallel synthesis: This involves the simultaneous synthesis of multiple compounds using a series of reaction vessels, each containing a different starting material or reagent. The resulting compounds are then screened for biological activity.
Diversity-oriented synthesis: This involves the generation of structurally diverse compounds by systematically varying the building blocks and reaction conditions used in the synthesis.
Combinatorial chemistry has played a significant role in drug discovery, particularly in the early stages of lead identification and optimization. It has enabled the rapid generation of large numbers of compounds with diverse structures and has led to the identification of several new drug candidates. However, the technique has limitations, including the need for efficient screening methods and the challenge of optimizing lead compounds with complex chemical structures.
Global Market:
The global combinatorial chemistry market is a rapidly growing industry that involves the application of combinatorial chemistry techniques in drug discovery and development. This market includes various products and services related to combinatorial chemistry, including instruments, software, and services.
According to a report by MarketsandMarkets, the global combinatorial chemistry market size is expected to reach USD 6.08 billion by 2025, growing at a compound annual growth rate (CAGR) of 7.6% from 2020 to 2025. The increasing demand for efficient drug discovery and development processes and the rising prevalence of chronic diseases are driving market growth.
The market is segmented based on product and service, technology, application, and region. Based on product and service, the market is segmented into instruments, software, and services. Based on technology, the market is segmented into solid-phase, solution-phase, and others. Based on application, the market is segmented into drug discovery and academic research.
North America dominated the global combinatorial chemistry market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical and biotechnology companies, well-established healthcare infrastructure, and increasing investment in research and development activities.
The key players operating in the global combinatorial chemistry market include Thermo Fisher Scientific, Inc., Merck KGaA, Albany Molecular Research Inc., IKA Works, Inc., and Shimadzu Corporation, among others.
Overall, the combinatorial chemistry market is expected to experience significant growth in the coming years, driven by technological advancements, increasing demand for efficient drug discovery and development processes, and the rising prevalence of chronic diseases.
Novel Drug Delivery Systems

Novel drug delivery systems (NDDS) are methods used to deliver drugs to their intended site of action in a controlled and targeted manner, with the aim of improving therapeutic efficacy and reducing side effects. These systems can be designed to control the release rate, duration, and site-specific targeting of drugs.
Some examples of NDDS include:
Liposomes: These are small vesicles composed of a lipid bilayer that can encapsulate drugs. Liposomes can protect the drug from degradation and clearance, and can improve its pharmacokinetics and biodistribution.
Nanoparticles: These are small particles (less than 100 nm in diameter) that can be composed of a variety of materials, including polymers, lipids, and metals. Nanoparticles can be designed to encapsulate drugs, and can be functionalized to target specific tissues or cells.
Microneedles: These are small, painless needles that can penetrate the outer layer of the skin to deliver drugs transdermally. Microneedles can be used to deliver drugs that are poorly absorbed through the skin, and can improve patient compliance.
Implants: These are small devices that can be implanted under the skin or in a specific tissue to deliver drugs over an extended period of time. Implants can provide sustained release of drugs and can reduce the need for frequent dosing.
Microfluidic systems: These are devices that can manipulate small volumes of fluids to create precise drug delivery systems. Microfluidic systems can be used to create complex drug delivery systems, such as multi-layered scaffolds or drug-releasing hydrogels.
NDDS have the potential to improve drug efficacy and patient outcomes, but they also present unique challenges. These include the need for precise control over drug release rates and dosing, the potential for immune reactions or toxicity, and the need for specialized manufacturing and testing procedures. Nonetheless, continued research in this area is expected to lead to the development of new and innovative drug delivery systems that can improve patient care.
Global Market:
The global novel drug delivery systems market is a rapidly growing industry that involves the development of innovative drug delivery systems to improve the efficacy, safety, and convenience of drug administration. This market includes various products and services related to novel drug delivery systems, including oral drug delivery, injectable drug delivery, transdermal drug delivery, and other advanced drug delivery systems.
According to a report by Grand View Research, Inc., the global novel drug delivery systems market size was valued at USD 322.95 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 7.4% from 2021 to 2028. The increasing prevalence of chronic diseases, growing demand for self-administration and home healthcare devices, and advancements in drug delivery technologies are driving market growth.
The market is segmented based on type, route of administration, and region. Based on type, the market is segmented into oral drug delivery, injectable drug delivery, transdermal drug delivery, and other advanced drug delivery systems. Based on the route of administration, the market is segmented into oral, parenteral, transdermal, and other routes.
North America dominated the global novel drug delivery systems market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical and biotechnology companies, well-established healthcare infrastructure, and increasing investments in research and development activities.
The key players operating in the global novel drug delivery systems market include 3M, Johnson & Johnson Services, Inc., Novartis AG, Pfizer Inc., and Roche Holding AG, among others.
Overall, the novel drug delivery systems market is expected to experience significant growth in the coming years, driven by technological advancements, increasing prevalence of chronic diseases, and growing demand for self-administration and home healthcare devices.
Formulations

Formulations refer to the process of developing a specific drug product by combining the active pharmaceutical ingredient (API) with other ingredients and excipients in a specific formulation to achieve the desired drug delivery characteristics. The goal of pharmaceutical formulations is to develop safe and effective dosage forms that can deliver the drug to the site of action in the desired concentration and time frame.
There are various types of drug formulations, including:
Solid dosage forms: These include tablets, capsules, powders, and granules. Solid dosage forms are convenient and easy to handle, and can provide sustained release of drugs.
Liquid dosage forms: These include solutions, suspensions, emulsions, and syrups. Liquid dosage forms are useful for drugs that are poorly soluble or unstable in solid form, and can provide faster onset of action.
Semi-solid dosage forms: These include creams, ointments, and gels. Semi-solid dosage forms are used for topical application and can provide prolonged drug release.
Parenteral dosage forms: These include injections, infusions, and implants. Parenteral dosage forms are used for drugs that cannot be administered orally and require immediate onset of action.
Inhalation dosage forms: These include inhalers and nebulizers. Inhalation dosage forms are used for drugs that need to be delivered directly to the lungs.
The choice of formulation depends on several factors, including the physicochemical properties of the drug, the target site of action, and patient preference. Formulation development involves a series of steps, including pre-formulation studies, formulation design, optimization, and stability testing.
Formulation development is an important aspect of drug development, as it can significantly impact the safety, efficacy, and patient compliance of a drug product.
Global Market:
The global formulations market is a rapidly growing industry that involves the development and production of pharmaceutical products in specific forms, such as tablets, capsules, creams, ointments, injectables, and inhalers. The market is driven by the increasing demand for patient-friendly drug products, the growing prevalence of chronic diseases, and the expanding pharmaceutical industry.
According to a report by ResearchAndMarkets.com, the global formulations market size was valued at USD 68.8 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 6.4% from 2021 to 2028. The increasing demand for innovative and patient-friendly drug products, the growing prevalence of chronic diseases, and the expanding pharmaceutical industry are driving market growth.
The market is segmented based on product type, route of administration, and region. Based on product type, the market is segmented into solid formulations, semi-solid formulations, liquid formulations, and others. Based on the route of administration, the market is segmented into oral, topical, parenteral, and others.
North America dominated the global formulations market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of major pharmaceutical and biotechnology companies, well-established healthcare infrastructure, and increasing investments in research and development activities.
The key players operating in the global formulations market include Pfizer Inc., Novartis AG, Roche Holding AG, Sanofi SA, and Johnson & Johnson, among others. These companies are investing heavily in research and development activities to introduce new and innovative formulations to the market and gain a competitive edge.
Overall, the formulations market is expected to experience significant growth in the coming years, driven by the increasing demand for patient-friendly drug products and the growing need for effective and safe drug delivery systems.
Natural Products

Natural products are chemical compounds that are derived from natural sources, such as plants, animals, or microorganisms. These compounds have been used for centuries in traditional medicine for the treatment of various diseases and conditions.
Natural products can be classified into several categories based on their chemical structure, including:
Alkaloids: These are nitrogen-containing compounds that are commonly found in plants. Examples include caffeine, morphine, and nicotine.
Terpenes: These are compounds that are commonly found in essential oils and are responsible for the characteristic odor and flavor of plants. Examples include menthol and limonene.
Flavonoids: These are compounds that are commonly found in fruits, vegetables, and herbs. Examples include quercetin and catechins.
Polyphenols: These are compounds that are commonly found in fruits, vegetables, and nuts. Examples include resveratrol and curcumin.
Natural products have been the source of many important drugs, including aspirin, penicillin, and taxol. Natural products have several advantages over synthetic drugs, including a wide range of chemical diversity, target specificity, and lower toxicity.
Natural products are still an important source of new drug leads, and several research programs are focused on the identification and development of natural products for the treatment of various diseases. However, the development of natural products as drugs can be challenging due to the limited supply, complex chemical structures, and difficulties in purification and standardization.
Global Market:
The global natural products market involves the production, distribution, and sale of products that are derived from natural sources, such as plants, animals, and minerals. These products are used for various purposes, including dietary supplements, personal care products, and medicinal purposes.
The natural products market is driven by the increasing demand for natural and organic products, the growing awareness about the health benefits of natural products, and the expanding consumer base in emerging markets. The market is also influenced by the growing trend towards sustainable and eco-friendly products.
According to a report by Zion Market Research, the global natural products market was valued at around USD 36.3 billion in 2020 and is expected to reach USD 58.5 billion by 2027, growing at a CAGR of around 6.9% between 2021 and 2027. The increasing demand for natural and organic products, the growing awareness about the health benefits of natural products, and the expanding consumer base in emerging markets are driving market growth.
The market is segmented based on product type, including food and beverage, personal care and cosmetics, pharmaceuticals, and others. The food and beverage segment dominated the market in 2020, accounting for the largest share of the global natural products market. The segment is driven by the growing demand for natural and organic food products, the increasing trend towards healthy eating habits, and the rising incidence of lifestyle diseases.
North America dominated the global natural products market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the high consumer awareness and demand for natural and organic products, the presence of major natural products companies, and the growing trend towards healthy lifestyles.
The key players operating in the global natural products market include Archer Daniels Midland Company, Cargill, Inc., BASF SE, DuPont de Nemours, Inc., and Koninklijke DSM N.V., among others. These companies are investing heavily in research and development activities to introduce new and innovative natural products to the market and gain a competitive edge.
Overall, the natural products market is expected to experience significant growth in the coming years, driven by the increasing demand for natural and organic products, the growing awareness about the health benefits of natural products, and the expanding consumer base in emerging markets.
Traditional Medicine

Traditional medicine refers to the medical practices that have been used for centuries in different cultures and regions of the world, outside of conventional or modern Western medicine. Traditional medicine is often based on indigenous knowledge and is used to prevent, diagnose, and treat various ailments and health conditions.
Traditional medicine can be classified into several categories, including:
Herbal medicine: This involves the use of plant-based remedies to treat various health conditions. Many herbs and plants have been used for medicinal purposes for centuries, and some have been shown to have pharmacological properties.
Acupuncture: This is a traditional Chinese medicine practice that involves the insertion of fine needles into specific points on the body to stimulate and regulate the flow of energy.
Ayurveda: This is an ancient Indian system of medicine that emphasizes the use of natural remedies, such as herbs, minerals, and massage, to maintain balance and harmony within the body.
Traditional African medicine: This includes the use of plant-based remedies, spiritual and cultural practices, and traditional healers to treat various health conditions.
Traditional medicine is often used in combination with modern Western medicine, and there is growing interest in the integration of traditional and modern medicine to provide comprehensive and culturally appropriate healthcare. However, the use of traditional medicine can also be associated with certain risks, such as the potential for misdiagnosis, interactions with modern medications, and contamination of herbal remedies. It is important to seek guidance from trained practitioners and to use traditional medicine in conjunction with modern medical care.
Global Market:
The global traditional medicine market involves the use of traditional and complementary medicine (T&CM) practices and products, such as herbal medicine, acupuncture, and traditional Ayurvedic medicine. These practices and products are based on the use of natural substances and traditional knowledge, and are widely used in many countries around the world.
The traditional medicine market is driven by the increasing demand for alternative and complementary medicine, the growing awareness about the health benefits of traditional medicine, and the expanding consumer base in emerging markets. The market is also influenced by the growing trend towards natural and holistic approaches to healthcare.
According to a report by Grand View Research, the global traditional medicine market size was valued at USD 78.8 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 6.7% from 2021 to 2028. The increasing adoption of alternative and complementary medicine, the growing prevalence of chronic diseases, and the expanding consumer base in emerging markets are driving market growth.
The market is segmented based on type, including herbal medicine, acupuncture, traditional Chinese medicine, and others. The herbal medicine segment dominated the market in 2020, accounting for the largest share of the global traditional medicine market. The segment is driven by the growing awareness about the health benefits of herbal medicine, the rising incidence of chronic diseases, and the growing demand for natural and holistic approaches to healthcare.
Asia Pacific dominated the global traditional medicine market in 2020, followed by North America and Europe. The dominance of Asia Pacific is attributed to the high adoption of traditional medicine practices, the presence of major traditional medicine companies, and the growing trend towards natural and holistic approaches to healthcare.
The key players operating in the global traditional medicine market include Pacific Pharmaceuticals Ltd., Beijing Tcmages Pharmaceutical Co. Ltd., China Beijing Tongrentang Group Co. Ltd., and Yunnan Baiyao Group Co. Ltd., among others. These companies are investing heavily in research and development activities to introduce new and innovative traditional medicine products to the market and gain a competitive edge.
Overall, the traditional medicine market is expected to experience significant growth in the coming years, driven by the increasing adoption of alternative and complementary medicine, the growing awareness about the health benefits of traditional medicine, and the expanding consumer base in emerging markets.
Drug Repurposing and Drug Repositioning

Drug repurposing, also known as drug repositioning or drug rediscovery, is the process of identifying new uses for existing drugs that have already been approved for the treatment of one condition. This approach is an alternative to traditional drug development, which can be costly and time-consuming.
Drug repurposing has several advantages over traditional drug development, including reduced costs and a shorter development timeline. In addition, because the safety profile of the drug is already established, the repurposed drug can be brought to market faster than a new drug.
Drug repurposing can be achieved through several approaches, including:
Target-based screening: This involves screening existing drugs against new drug targets.
Phenotypic screening: This involves screening existing drugs against disease models to identify drugs with new therapeutic uses.
Structure-based drug design: This involves using the three-dimensional structure of the drug and its target to identify new uses.
Computational approaches: This involves using computer algorithms and databases to identify new uses for existing drugs.
Drug repurposing has been successful in identifying new uses for several drugs. For example, the drug sildenafil was originally developed for the treatment of angina but was later repurposed for the treatment of erectile dysfunction. Similarly, the drug thalidomide, which was originally used as a sedative, was repurposed for the treatment of leprosy and multiple myeloma.
Overall, drug repurposing is a promising approach to drug development that can significantly reduce the time and cost of drug development and increase the availability of new treatments for patients.
Global Market:
The global drug repurposing and drug repositioning market involves the discovery, development, and commercialization of new therapeutic uses for existing drugs. This approach allows for the identification of new indications, improved efficacy, and reduced development costs and timelines for existing drugs, which have already undergone safety and toxicity testing.
The drug repurposing and repositioning market is driven by the increasing demand for more efficient and cost-effective drug development approaches, the need for new therapies for diseases with limited treatment options, and the growing availability of large-scale data and computational tools for drug discovery.
According to a report by MarketsandMarkets, the global drug repurposing market size is expected to grow from USD 24.2 billion in 2020 to USD 31.0 billion by 2025, at a compound annual growth rate (CAGR) of 5.1% during the forecast period. The increasing demand for more efficient drug development approaches, the need for new therapies for diseases with limited treatment options, and the growing availability of large-scale data and computational tools for drug discovery are driving market growth.
The market is segmented based on application, including oncology, cardiovascular diseases, neurological disorders, and others. The oncology segment dominated the market in 2020, accounting for the largest share of the global drug repurposing market. The segment is driven by the high prevalence of cancer, the need for more effective therapies, and the availability of a large number of existing drugs for repurposing.
North America dominated the global drug repurposing market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of pharmaceutical companies, the high healthcare expenditure, and the growing adoption of advanced drug discovery technologies.
The key players operating in the global drug repurposing and repositioning market include Novartis AG, Pfizer Inc., Sanofi S.A., AstraZeneca plc, and GlaxoSmithKline plc, among others. These companies are investing heavily in research and development activities to identify new therapeutic uses for existing drugs and gain a competitive edge.
Overall, the drug repurposing and repositioning market is expected to experience significant growth in the coming years, driven by the increasing demand for more efficient drug development approaches, the need for new therapies for diseases with limited treatment options, and the growing availability of large-scale data and computational tools for drug discovery.
Precision Medicine

Precision medicine is an approach to healthcare that takes into account an individual's genetic, environmental, and lifestyle factors to tailor medical treatments to the specific needs of that individual. This approach recognizes that different patients may respond differently to the same medical treatment, and seeks to provide more personalized and effective care.
Precision medicine involves the use of various technologies and approaches, including genomics, proteomics, metabolomics, and bioinformatics, to identify the unique characteristics of each patient and develop customized treatment plans. For example, genetic testing can help identify genetic mutations or variations that may increase the risk of certain diseases or affect how a patient responds to certain drugs. This information can then be used to develop personalized treatment plans that are tailored to the patient's individual needs.
Precision medicine has the potential to revolutionize healthcare by providing more effective, personalized, and cost-effective treatments. It can also help reduce the risk of adverse drug reactions, improve patient outcomes, and reduce healthcare costs by avoiding unnecessary treatments and procedures.
However, the implementation of precision medicine is not without its challenges, including the need for large-scale data analysis, standardization of data collection and analysis, and ethical and legal considerations related to the use of patient data. Despite these challenges, precision medicine is rapidly advancing, and it is expected to play an increasingly important role in the future of healthcare.
Global Market:
Precision medicine is an emerging approach to disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person. It involves the use of advanced technologies and analytics to understand the underlying mechanisms of disease and to develop targeted therapies and diagnostics for specific patient subpopulations.
The global precision medicine market is expected to grow significantly in the coming years, driven by factors such as increasing demand for personalized healthcare, the growing adoption of advanced technologies in healthcare, and the need to reduce healthcare costs by improving treatment outcomes.
According to a report by MarketsandMarkets, the global precision medicine market size is expected to grow from USD 66.7 billion in 2020 to USD 110.2 billion by 2025, at a compound annual growth rate (CAGR) of 10.5% during the forecast period. The market is segmented based on technology, application, and end user.
Based on technology, the market is segmented into genomics, proteomics, metabolomics, epigenetics, and others. The genomics segment dominated the market in 2020, accounting for the largest share of the global precision medicine market. The segment is driven by the increasing use of next-generation sequencing (NGS) technologies for sequencing DNA and RNA.
Based on application, the market is segmented into oncology, immunology, neurology, cardiology, infectious diseases, and others. The oncology segment dominated the market in 2020, accounting for the largest share of the global precision medicine market. The segment is driven by the increasing prevalence of cancer and the need for more effective and personalized therapies.
Based on end user, the market is segmented into pharmaceutical and biotechnology companies, diagnostic companies, healthcare IT companies, and clinical laboratories. The pharmaceutical and biotechnology companies segment dominated the market in 2020, accounting for the largest share of the global precision medicine market. The segment is driven by the increasing investment by pharmaceutical companies in precision medicine research and development.
North America dominated the global precision medicine market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of pharmaceutical and biotechnology companies, the high healthcare expenditure, and the growing adoption of advanced technologies in healthcare.
The key players operating in the global precision medicine market include Thermo Fisher Scientific Inc., Illumina Inc., QIAGEN N.V., Roche Holding AG, and Novartis AG, among others. These companies are investing heavily in research and development activities to develop targeted therapies and diagnostics for specific patient subpopulations and gain a competitive edge.
Overall, the precision medicine market is expected to experience significant growth in the coming years, driven by the increasing demand for personalized healthcare, the growing adoption of advanced technologies in healthcare, and the need to reduce healthcare costs by improving treatment outcomes.
Personalized Therapies

Personalized therapies are treatments that are tailored to an individual's unique characteristics, such as their genetics, lifestyle, and medical history. The goal of personalized therapies is to provide more effective and targeted treatments that are better suited to the specific needs of each patient. This approach contrasts with traditional "one-size-fits-all" approaches to medicine, which may not take into account individual differences in patients' biology or health status.
In recent years, personalized therapies have gained increasing attention in various fields of medicine, including oncology, cardiology, and neurology. For example, personalized cancer therapies may involve genetic testing to identify specific mutations in a patient's tumor cells, which can help guide the selection of targeted therapies that are more likely to be effective. Similarly, personalized cardiovascular therapies may involve lifestyle modifications, such as dietary changes and exercise programs, in addition to medication and surgical interventions.
Overall, personalized therapies have the potential to improve patient outcomes and reduce healthcare costs by minimizing the use of ineffective treatments and optimizing the use of more targeted interventions. However, personalized therapies also require a significant amount of research and testing to determine the most effective treatment strategies for each patient.
Global Market:
Personalized therapies refer to treatments that are tailored to the individual characteristics of each patient, such as their genetic makeup, disease subtype, or other factors. This approach to treatment aims to improve patient outcomes and reduce adverse events by targeting therapies to the specific needs of each patient.
The global personalized therapies market is expected to grow significantly in the coming years, driven by factors such as increasing demand for personalized healthcare, advancements in genetic sequencing technologies, and the need to reduce healthcare costs by improving treatment outcomes.
According to a report by MarketsandMarkets, the global personalized therapies market size is expected to reach USD 2.4 trillion by 2025, at a compound annual growth rate (CAGR) of 10.5% during the forecast period. The market is segmented based on product, application, and end user.
Based on product, the market is segmented into cell and gene therapies, personalized medical devices, personalized medicines, and personalized nutrition and wellness. The personalized medicines segment dominated the market in 2020, accounting for the largest share of the global personalized therapies market. The segment is driven by the increasing use of genomic testing and other diagnostic tools to identify patients who are likely to benefit from targeted therapies.
Based on application, the market is segmented into oncology, neurology, cardiology, infectious diseases, and others. The oncology segment dominated the market in 2020, accounting for the largest share of the global personalized therapies market. The segment is driven by the increasing prevalence of cancer and the need for more effective and personalized therapies.
Based on end user, the market is segmented into hospitals and clinics, diagnostic laboratories, and research institutions. The hospitals and clinics segment dominated the market in 2020, accounting for the largest share of the global personalized therapies market. The segment is driven by the increasing adoption of personalized therapies in hospital and clinic settings.
North America dominated the global personalized therapies market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of pharmaceutical and biotechnology companies, the high healthcare expenditure, and the growing adoption of personalized healthcare in the region.
The key players operating in the global personalized therapies market include Thermo Fisher Scientific Inc., Illumina Inc., QIAGEN N.V., Roche Holding AG, and Novartis AG, among others. These companies are investing heavily in research and development activities to develop targeted therapies and diagnostics for specific patient subpopulations and gain a competitive edge.
Overall, the personalized therapies market is expected to experience significant growth in the coming years, driven by the increasing demand for personalized healthcare, advancements in genetic sequencing technologies, and the need to reduce healthcare costs by improving treatment outcomes.
Drug Development and Clinical Trials

Drug development is a complex process that involves a series of stages, from the initial discovery of a potential drug target to the eventual marketing and distribution of a new drug. One of the critical steps in drug development is the conduct of clinical trials, which are designed to evaluate the safety, efficacy, and optimal dosing of a new drug in human subjects.
Clinical trials typically involve a series of phases, starting with small-scale safety studies in healthy volunteers and progressing to larger-scale efficacy studies in patients with the targeted disease or condition. Phase 1 trials are typically designed to evaluate safety and dose-response relationships, while phase 2 and 3 trials aim to establish the efficacy and safety of the drug in the target population.
During clinical trials, patients are typically randomized to receive either the new drug or a placebo (a dummy pill that looks identical to the active drug) to determine whether the drug is more effective than no treatment or standard of care. The trial is usually blinded, meaning that the patients and the researchers do not know which patients are receiving the active drug or placebo until the trial is complete, to reduce the risk of bias.
Clinical trials are also subject to regulatory oversight by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), which evaluate the safety and efficacy data from the trials to determine whether the drug should be approved for marketing and distribution. This regulatory process typically involves a rigorous review of the clinical trial data, as well as preclinical data on the drug's safety and efficacy in animals.
Overall, drug development and clinical trials are complex and expensive processes that involve many stakeholders, including pharmaceutical companies, academic researchers, regulatory agencies, and patients. However, the ultimate goal is to develop safe and effective drugs that can improve patient outcomes and quality of life.
Global Market:
Drug development and clinical trials are essential components of the pharmaceutical industry, allowing new drugs to be tested for safety and efficacy before they can be approved for use by regulatory authorities. The global drug development and clinical trials market includes a range of services and technologies designed to support drug development, including clinical trial management, data management, and regulatory affairs.
The global drug development and clinical trials market is expected to grow significantly in the coming years, driven by factors such as the increasing demand for new and innovative therapies, the rising prevalence of chronic diseases, and advancements in clinical trial technology.
According to a report by Market Research Future, the global drug development and clinical trials market is expected to reach USD 65.2 billion by 2025, at a compound annual growth rate (CAGR) of 6.8% during the forecast period. The market is segmented based on phase, service, and region.
Based on phase, the market is segmented into phase I, phase II, phase III, and phase IV/post-marketing surveillance. The phase III segment dominated the market in 2020, accounting for the largest share of the global drug development and clinical trials market. The segment is driven by the increasing number of drugs in late-stage development and the need to generate robust data on safety and efficacy.
Based on service, the market is segmented into clinical trial management, data management, regulatory affairs, medical writing and publishing, clinical monitoring, site management, and others. The clinical trial management segment dominated the market in 2020, accounting for the largest share of the global drug development and clinical trials market. The segment is driven by the increasing demand for clinical trial management services and the need for efficient and effective trial design and execution.
North America dominated the global drug development and clinical trials market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of pharmaceutical and biotechnology companies, the high healthcare expenditure, and the favorable regulatory environment.
The key players operating in the global drug development and clinical trials market include IQVIA Holdings Inc., PAREXEL International Corporation, Charles River Laboratories International Inc., ICON plc, and Covance Inc., among others. These companies are investing heavily in research and development activities to develop new and innovative clinical trial technologies and gain a competitive edge.
Overall, the drug development and clinical trials market is expected to experience significant growth in the coming years, driven by the increasing demand for new and innovative therapies, the rising prevalence of chronic diseases, and advancements in clinical trial technology.
Biomarker Discovery and Development

Biomarkers are measurable indicators of biological processes or conditions that can be used to diagnose, monitor, or predict the course of disease. Biomarkers can include genetic, epigenetic, proteomic, or metabolomic markers, among others. The discovery and development of biomarkers is a critical step in the development of personalized medicine, as biomarkers can be used to identify patients who are more likely to respond to a particular treatment or who are at higher risk for a specific disease.
Biomarker discovery involves identifying and validating new biomarkers that are associated with specific disease states or treatment outcomes. This process typically involves a combination of preclinical and clinical studies, including studies of biomarker expression in tissue samples, studies of biomarker levels in blood or other bodily fluids, and studies of biomarker response to specific treatments or interventions.
Once potential biomarkers have been identified, they must be validated in larger clinical studies to demonstrate their utility in predicting disease outcomes or treatment responses. This validation process may involve studies in diverse patient populations or studies in combination with other biomarkers or clinical parameters.
The development of biomarkers can also involve the development of diagnostic or prognostic tests that can be used to guide treatment decisions or monitor disease progression. These tests may involve the use of imaging techniques, such as magnetic resonance imaging (MRI) or positron emission tomography (PET), or the use of blood or tissue-based tests to measure biomarker levels.
Overall, the discovery and development of biomarkers is a critical step in the development of personalized medicine, as it can help to identify patients who are more likely to benefit from a particular treatment and to develop more targeted and effective therapies for specific disease states.
Global Market:
Biomarker discovery and development is a critical component of the pharmaceutical and biotechnology industries, as it involves the identification and validation of biomarkers for various diseases and conditions, which can be used to improve diagnosis, monitor disease progression, and predict response to therapy. The global biomarker discovery and development market includes a range of services and technologies designed to support biomarker research, such as assay development, biomarker validation, and biomarker testing.
The global biomarker discovery and development market is expected to grow significantly in the coming years, driven by factors such as the increasing demand for personalized medicine, the rising prevalence of chronic diseases, and advancements in biomarker technology.
According to a report by Market Research Future, the global biomarker discovery and development market is expected to reach USD 69.09 billion by 2025, at a compound annual growth rate (CAGR) of 13.44% during the forecast period. The market is segmented based on type, application, disease area, and region.
Based on type, the market is segmented into safety biomarkers, efficacy biomarkers, and validation biomarkers. The efficacy biomarkers segment dominated the market in 2020, accounting for the largest share of the global biomarker discovery and development market. The segment is driven by the increasing demand for biomarkers that can predict response to therapy and guide treatment decisions.
Based on application, the market is segmented into diagnostics development, drug discovery and development, personalized medicine, and others. The diagnostics development segment dominated the market in 2020, accounting for the largest share of the global biomarker discovery and development market. The segment is driven by the increasing demand for biomarkers that can improve diagnosis and monitoring of diseases.
Based on disease area, the market is segmented into oncology, cardiovascular diseases, neurology, immunology, and others. The oncology segment dominated the market in 2020, accounting for the largest share of the global biomarker discovery and development market. The segment is driven by the increasing demand for biomarkers that can improve diagnosis and monitoring of cancer.
North America dominated the global biomarker discovery and development market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of pharmaceutical and biotechnology companies, the high healthcare expenditure, and the favorable regulatory environment.
The key players operating in the global biomarker discovery and development market include Thermo Fisher Scientific Inc., QIAGEN, F. Hoffmann-La Roche Ltd., Bio-Rad Laboratories, Inc., and Agilent Technologies, Inc., among others. These companies are investing heavily in research and development activities to develop new and innovative biomarker technologies and gain a competitive edge.
Overall, the biomarker discovery and development market is expected to experience significant growth in the coming years, driven by the increasing demand for personalized medicine, the rising prevalence of chronic diseases, and advancements in biomarker technology.
Biologics and Biosimilars

Biologics are complex drugs derived from living organisms, such as proteins, antibodies, or nucleic acids. They are used to treat a wide range of diseases, including cancer, autoimmune disorders, and inflammatory conditions.
Biosimilars, on the other hand, are biologic drugs that are similar to an already approved reference biologic drug, also known as the originator. Biosimilars are not identical to the originator, but they are highly similar in terms of quality, safety, and efficacy.
Biosimilars are developed using a rigorous scientific process that involves comparing them to the reference biologic drug in terms of their structure, function, and clinical outcomes. They undergo extensive testing and evaluation before they are approved for use by regulatory agencies.
Biosimilars can offer significant benefits to patients and healthcare systems by increasing access to biologic therapies and reducing healthcare costs. They also promote competition in the biologics market, which can lead to lower prices and increased innovation.
However, it's important to note that biosimilars are not interchangeable with their reference biologic drugs. Interchangeability means that the biosimilar can be substituted for the reference biologic drug without the involvement of the prescribing healthcare provider. Interchangeability is determined by regulatory agencies based on specific criteria, and currently, there are very few biosimilars that have been granted interchangeable status.
Global Market:
Biologics and biosimilars are an important and growing segment of the pharmaceutical industry, representing a range of innovative drugs and therapies for the treatment of various diseases and conditions. Biologics are complex molecules produced using living cells, while biosimilars are highly similar copies of biologics that have been approved for use by regulatory authorities.
The global biologics and biosimilars market is expected to grow significantly in the coming years, driven by factors such as the increasing prevalence of chronic diseases, the growing demand for biologics and biosimilars, and advancements in biotechnology and manufacturing processes.
According to a report by Grand View Research, the global biologics and biosimilars market was valued at USD 255.1 billion in 2020 and is expected to reach USD 523.0 billion by 2028, at a compound annual growth rate (CAGR) of 9.5% during the forecast period. The market is segmented based on product type, application, and region.
Based on product type, the market is segmented into monoclonal antibodies, vaccines, recombinant hormones, growth factors, and others. Monoclonal antibodies dominated the market in 2020, accounting for the largest share of the global biologics and biosimilars market. The segment is driven by the increasing use of monoclonal antibodies in the treatment of cancer and autoimmune diseases.
Based on application, the market is segmented into oncology, immunology, diabetes, infectious diseases, and others. Oncology dominated the market in 2020, accounting for the largest share of the global biologics and biosimilars market. The segment is driven by the increasing use of biologics and biosimilars in the treatment of various types of cancer.
North America dominated the global biologics and biosimilars market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of biotechnology and pharmaceutical companies, the high healthcare expenditure, and the favorable regulatory environment.
The key players operating in the global biologics and biosimilars market include AbbVie Inc., Amgen Inc., Biogen Inc., Pfizer Inc., and Roche Holding AG, among others. These companies are investing heavily in research and development activities to develop new and innovative biologics and biosimilars and gain a competitive edge.
Overall, the biologics and biosimilars market is expected to experience significant growth in the coming years, driven by the increasing prevalence of chronic diseases, the growing demand for biologics and biosimilars, and advancements in biotechnology and manufacturing processes.
Vaccines and Immunotherapies

Vaccines and immunotherapies are both types of treatments that can help boost the body's immune system and protect against diseases.
Vaccines are typically given to healthy individuals to prevent infections from occurring in the first place. They work by introducing a weakened or dead form of the disease-causing organism or a piece of the organism (such as a protein) to the immune system, which then mounts an immune response and creates memory cells that remember how to fight the organism in the future. This means that if the person is exposed to the disease later, their immune system is prepared to fight it off before it can cause illness.
Immunotherapies, on the other hand, are used to treat diseases that have already developed, such as cancer or autoimmune disorders. They work by boosting the body's natural defenses to help fight off the disease. There are several types of immunotherapies, including monoclonal antibodies, checkpoint inhibitors, and cell-based therapies, each with different mechanisms of action.
While vaccines are generally given to healthy individuals to prevent disease, immunotherapies are used to treat individuals who already have a disease. However, there are some vaccines that can also be used as immunotherapies. For example, the Bacillus Calmette-Guérin (BCG) vaccine, which is used to protect against tuberculosis, has also been used as an immunotherapy for bladder cancer.
Overall, both vaccines and immunotherapies are important tools in protecting and improving public health. Vaccines can prevent many diseases from occurring in the first place, while immunotherapies can help treat diseases that are already present.
Global Market:
Vaccines and immunotherapies are critical components of the healthcare industry, helping to prevent and treat a wide range of infectious diseases and conditions. Vaccines are used to prevent diseases by stimulating the body's immune system to produce antibodies that can fight off harmful pathogens. Immunotherapies, on the other hand, are designed to treat diseases by enhancing or modifying the body's immune response to specific targets.
The global vaccines and immunotherapies market is expected to grow significantly in the coming years, driven by factors such as the increasing prevalence of infectious diseases, the growing demand for vaccines and immunotherapies, and advancements in technology and manufacturing processes.
According to a report by Grand View Research, the global vaccines and immunotherapies market was valued at USD 172.5 billion in 2020 and is expected to reach USD 310.0 billion by 2028, at a compound annual growth rate (CAGR) of 7.5% during the forecast period. The market is segmented based on product type, application, and region.
Based on product type, the market is segmented into vaccines and immunotherapies. Vaccines dominated the market in 2020, accounting for the largest share of the global vaccines and immunotherapies market. The segment is driven by the increasing demand for vaccines to prevent infectious diseases such as COVID-19, influenza, and hepatitis.
Based on application, the market is segmented into infectious diseases, cancer, and others. Infectious diseases dominated the market in 2020, accounting for the largest share of the global vaccines and immunotherapies market. The segment is driven by the increasing prevalence of infectious diseases such as COVID-19, tuberculosis, and malaria.
North America dominated the global vaccines and immunotherapies market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of biotechnology and pharmaceutical companies, the high healthcare expenditure, and the favorable regulatory environment.
The key players operating in the global vaccines and immunotherapies market include GlaxoSmithKline plc, Johnson & Johnson, Merck & Co., Inc., Novartis International AG, and Pfizer Inc., among others. These companies are investing heavily in research and development activities to develop new and innovative vaccines and immunotherapies and gain a competitive edge.
Overall, the vaccines and immunotherapies market is expected to experience significant growth in the coming years, driven by the increasing prevalence of infectious diseases, the growing demand for vaccines and immunotherapies, and advancements in technology and manufacturing processes.
Steam Cells and Regenerative Medicine

Stem cells are a type of cell that can differentiate into many different types of cells in the body, such as muscle, nerve, or blood cells. They are therefore of great interest in regenerative medicine, which is a field that aims to use stem cells and other techniques to replace or repair damaged or diseased tissue.
There are several types of stem cells, including embryonic stem cells, which are derived from embryos, and adult stem cells, which are found in various tissues in the body, such as bone marrow, fat, or muscle. In addition, induced pluripotent stem cells (iPSCs) can be generated from adult cells that have been reprogrammed to have stem cell-like properties.
Regenerative medicine approaches using stem cells can involve transplanting stem cells directly into damaged tissue, or using stem cells to create new tissue in the laboratory, which can then be transplanted into the body. In addition, stem cells can be used to create new drugs or therapies that target specific diseases or conditions.
Stem cell-based therapies have shown promise in treating a variety of conditions, such as heart disease, spinal cord injuries, and diabetes. However, there are also challenges associated with stem cell-based therapies, including the potential for rejection by the immune system and the risk of tumor formation.
Overall, stem cells and regenerative medicine hold great potential for treating a wide range of diseases and conditions, but further research is needed to fully understand their potential and how to optimize their use for safe and effective therapies.
Global Market:
Stem cells and regenerative medicine are rapidly advancing fields in healthcare that aim to develop innovative therapies for a wide range of diseases and conditions by using the body's own cells to repair or replace damaged tissue. Stem cells are undifferentiated cells that have the ability to differentiate into various types of cells in the body, making them a promising tool for regenerative medicine.
The global stem cells and regenerative medicine market is expected to grow significantly in the coming years, driven by factors such as the increasing prevalence of chronic diseases, the growing demand for regenerative medicine therapies, and advancements in technology and research.
According to a report by Market Research Future, the global stem cells and regenerative medicine market was valued at USD 10.3 billion in 2020 and is expected to reach USD 51.4 billion by 2028, at a compound annual growth rate (CAGR) of 22.36% during the forecast period. The market is segmented based on product type, application, and region.
Based on product type, the market is segmented into cell therapies, gene therapies, tissue engineering, and others. Cell therapies dominated the market in 2020, accounting for the largest share of the global stem cells and regenerative medicine market. The segment is driven by the increasing demand for cell-based therapies to treat a wide range of diseases and conditions, including cancer, cardiovascular diseases, and autoimmune disorders.
Based on application, the market is segmented into orthopedic and musculoskeletal disorders, cardiovascular diseases, oncology, dermatology, and others. Orthopedic and musculoskeletal disorders dominated the market in 2020, accounting for the largest share of the global stem cells and regenerative medicine market. The segment is driven by the increasing prevalence of orthopedic and musculoskeletal disorders, such as osteoarthritis, and the growing demand for regenerative medicine therapies to treat these conditions.
North America dominated the global stem cells and regenerative medicine market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of biotechnology and pharmaceutical companies, the high healthcare expenditure, and the favorable regulatory environment.
The key players operating in the global stem cells and regenerative medicine market include Novartis AG, Spark Therapeutics LLC, Vericel Corporation, Stryker Corporation, and Zimmer Biomet Holdings, Inc., among others. These companies are investing heavily in research and development activities to develop new and innovative stem cell-based therapies and gain a competitive edge.
Overall, the stem cells and regenerative medicine market is expected to experience significant growth in the coming years, driven by the increasing prevalence of chronic diseases, the growing demand for regenerative medicine therapies, and advancements in technology and research.
Drug Safety and Pharmacovigilance

Drug safety and pharmacovigilance are important aspects of the pharmaceutical industry and healthcare system that focus on ensuring the safe and effective use of drugs.
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. It involves monitoring the safety of drugs before and after they are approved for use, collecting and analyzing data on adverse reactions, and taking appropriate measures to minimize risks and maximize benefits.
Pharmacovigilance is important because all drugs have the potential to cause adverse effects, even those that are well-tested and approved for use. By monitoring drug safety and taking action to minimize risks, healthcare providers and regulators can help ensure that patients receive the safest and most effective treatments possible.
Drug safety is also a key consideration in the development and approval of new drugs. Before a drug can be approved for use, it must go through a series of clinical trials to test its safety and efficacy. Regulatory agencies such as the FDA and EMA also require ongoing monitoring of drugs after they are approved for use to ensure that any new safety concerns are identified and addressed in a timely manner.
In addition to regulatory agencies, pharmaceutical companies are also responsible for monitoring the safety of their drugs and reporting any adverse events to the appropriate authorities. They are required to have pharmacovigilance systems in place to ensure that they are collecting and reporting accurate data on drug safety.
Overall, drug safety and pharmacovigilance are critical components of the healthcare system that help ensure that drugs are used safely and effectively to improve patient outcomes.
Global Market:
Drug safety and pharmacovigilance are important aspects of the healthcare industry that aim to ensure the safe use of drugs and protect patients from any potential adverse effects of drugs. Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems.
The global drug safety and pharmacovigilance market is expected to grow significantly in the coming years, driven by factors such as the increasing number of adverse drug reactions (ADRs), the growing demand for drug safety and pharmacovigilance services, and advancements in technology and research.
According to a report by Market Research Future, the global drug safety and pharmacovigilance market was valued at USD 4.85 billion in 2020 and is expected to reach USD 8.25 billion by 2028, at a compound annual growth rate (CAGR) of 6.7% during the forecast period. The market is segmented based on service provider, type, and region.
Based on service provider, the market is segmented into in-house and contract outsourcing. Contract outsourcing dominated the market in 2020, accounting for the largest share of the global drug safety and pharmacovigilance market. The segment is driven by the increasing demand for outsourcing pharmacovigilance activities by pharmaceutical companies to reduce costs and focus on core activities.
Based on type, the market is segmented into spontaneous reporting, targeted spontaneous reporting, cohort event monitoring, and intensified ADR reporting. Spontaneous reporting dominated the market in 2020, accounting for the largest share of the global drug safety and pharmacovigilance market. The segment is driven by the increasing number of ADRs reported through spontaneous reporting systems and the growing awareness among healthcare professionals and patients about the importance of reporting ADRs.
North America dominated the global drug safety and pharmacovigilance market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of pharmaceutical and biotechnology companies, the high healthcare expenditure, and the favorable regulatory environment.
The key players operating in the global drug safety and pharmacovigilance market include Accenture, Boehringer Ingelheim, Bristol-Myers Squibb, Cognizant, Covance, IQVIA, PPD, Pfizer, and QuintilesIMS, among others. These companies are investing heavily in research and development activities to develop new and innovative drug safety and pharmacovigilance solutions and gain a competitive edge.
Overall, the drug safety and pharmacovigilance market is expected to experience significant growth in the coming years, driven by the increasing number of ADRs, the growing demand for drug safety and pharmacovigilance services, and advancements in technology and research.
Pharmacogenomics

Pharmacogenomics is the study of how a person's genetic makeup affects their response to drugs. It involves the use of genetic information to optimize drug therapy, predict drug responses, and minimize adverse reactions.
The human genome contains thousands of genes that can affect how the body metabolizes and responds to drugs. By identifying genetic variations that are associated with drug response, pharmacogenomics can help healthcare providers tailor drug therapy to individual patients and minimize the risk of adverse drug reactions.
Pharmacogenomics can be used in a variety of ways, including:
Pharmacogenomics has the potential to improve drug therapy and reduce healthcare costs by minimizing the need for trial and error in drug selection and dosing. However, there are also challenges associated with pharmacogenomics, including the need for standardized testing and interpretation of genetic data, and the potential for disparities in access to testing and personalized therapies.
Overall, pharmacogenomics is an important field that has the potential to improve the safety and effectiveness of drug therapy by tailoring treatments to individual patients based on their genetic makeup.
Global Market:
Pharmacogenomics is the study of how an individual's genetic makeup affects their response to drugs. The goal of pharmacogenomics is to develop personalized medicine that is tailored to an individual's genetic profile, leading to more effective treatments with fewer side effects.
The global pharmacogenomics market is expected to grow significantly in the coming years, driven by factors such as increasing demand for personalized medicine, advances in genomics research, and the growing need for more efficient drug development and clinical trials.
According to a report by Market Research Future, the global pharmacogenomics market was valued at USD 5.9 billion in 2020 and is expected to reach USD 11.1 billion by 2028, at a compound annual growth rate (CAGR) of 8.6% during the forecast period. The market is segmented based on technology, application, and region.
Based on technology, the market is segmented into polymerase chain reaction (PCR), sequencing, microarray, and others. Sequencing dominated the market in 2020, accounting for the largest share of the global pharmacogenomics market. The segment is driven by the growing adoption of next-generation sequencing (NGS) technologies in pharmacogenomics research.
Based on application, the market is segmented into oncology, cardiovascular, neurological, and others. Oncology dominated the market in 2020, accounting for the largest share of the global pharmacogenomics market. The segment is driven by the increasing focus on precision medicine in cancer treatment and the growing demand for companion diagnostics.
North America dominated the global pharmacogenomics market in 2020, followed by Europe and the Asia Pacific. The dominance of North America is attributed to the presence of a large number of pharmaceutical and biotechnology companies, increasing government funding for genomics research, and favorable regulatory environment.
The key players operating in the global pharmacogenomics market include Abbott Laboratories, Agilent Technologies, Inc., Bayer AG, F. Hoffmann-La Roche Ltd., Illumina, Inc., Myriad Genetics, Inc., Qiagen NV, Thermo Fisher Scientific Inc., and others. These companies are investing heavily in research and development activities to develop new and innovative pharmacogenomics solutions and gain a competitive edge.
Overall, the pharmacogenomics market is expected to experience significant growth in the coming years, driven by the increasing demand for personalized medicine, advances in genomics research, and the growing need for more efficient drug development and clinical trials.
Toxicology

Toxicology is the study of the harmful effects of chemical, physical, and biological agents on living organisms. It is a multidisciplinary field that involves the study of toxic substances, their effects on biological systems, and the mechanisms by which they cause harm.
Toxicologists use a variety of methods to assess the toxicity of chemicals, including animal studies, in vitro testing, and computer modeling. They also study the way in which toxic substances enter the body, how they are metabolized and eliminated, and how they interact with biological systems.
Toxicology has many practical applications, including:
Toxicology is a critical field that plays an important role in protecting public health and the environment. By identifying and understanding the harmful effects of toxic substances, toxicologists can help prevent and mitigate the risks associated with exposure to these substances.
Global Market:
The global market for toxicology is expected to continue to grow in the coming years. The demand for toxicology services is being driven by several factors, including the increasing awareness of the health hazards of chemicals and environmental pollutants, the need to ensure the safety of drugs and other consumer products, and the growing emphasis on regulatory compliance.
According to a report by MarketsandMarkets, the global toxicology services market is projected to reach $27.36 billion by 2021, growing at a compound annual growth rate (CAGR) of 12.2% from 2016 to 2021. North America is expected to hold the largest share of the market during this period, followed by Europe and Asia-Pacific.
The market is divided into several segments, including in vitro toxicology testing, toxicity testing services, regulatory toxicology services, and others. In vitro toxicology testing is expected to experience the fastest growth due to its advantages over traditional animal testing methods, including cost savings, reduced ethical concerns, and the ability to test large numbers of compounds simultaneously.
In addition to the traditional toxicology services market, there is also a growing demand for personalized medicine and pharmacogenomics, which involve the use of genetic information to tailor drug treatments to individual patients. This is expected to create new opportunities for toxicology services companies that specialize in genetic toxicology testing and related services.
Overall, the toxicology market is expected to continue to grow in the coming years as companies seek to ensure the safety and regulatory compliance of their products and as the demand for personalized medicine and pharmacogenomics increases.
Rare Diseases

Rare diseases are defined as diseases that affect a small number of people, usually fewer than 200,000 individuals in the United States or fewer than 1 in 2,000 people in Europe. However, the definition of a rare disease varies by region and country.
There are more than 7,000 rare diseases, and they can affect people of all ages and backgrounds. Many rare diseases are genetic, meaning that they are caused by mutations or changes in genes. However, not all rare diseases are genetic.
People with rare diseases often face significant challenges in getting an accurate diagnosis, accessing appropriate medical care, and finding effective treatments. This is due in part to the lack of understanding and awareness of rare diseases among healthcare providers and the general public.
Despite the challenges, there has been significant progress in the diagnosis and treatment of rare diseases in recent years. Advances in genetics and molecular biology have made it possible to identify the underlying genetic causes of many rare diseases, which has led to the development of new treatments and therapies.
In addition, there are many patient advocacy groups and organizations that work to raise awareness of rare diseases, provide support and resources for patients and families, and promote research into new treatments and cures.
Overall, rare diseases represent a significant public health challenge, but with continued research, advocacy, and support, there is hope for improved outcomes and quality of life for people affected by rare diseases.
Global Market:
The global market for rare diseases is a rapidly growing segment of the pharmaceutical industry, with an increasing number of treatments and therapies being developed to address these often-debilitating conditions. According to a report by ResearchAndMarkets, the global rare disease market is expected to grow at a compound annual growth rate (CAGR) of 11.6% from 2021 to 2026, reaching a value of $575.5 billion by the end of the forecast period.
There are over 7,000 identified rare diseases, which collectively affect an estimated 400 million people worldwide. While each individual rare disease may only affect a small number of people, the total number of individuals affected is significant, and the need for effective treatments is high. In recent years, there has been a growing focus on developing treatments for rare diseases, with regulatory agencies providing incentives and fast-track approvals for these types of therapies.
The market for rare disease treatments is divided into several segments, including gene therapy, enzyme replacement therapy, biologics, and small molecule drugs. Gene therapy is expected to experience the fastest growth, driven by advances in gene-editing technologies and the potential for long-term, curative treatments for genetic diseases.
North America and Europe are the largest markets for rare disease treatments, with a significant portion of the research and development taking place in these regions. However, there is also growing interest in developing treatments for rare diseases in emerging markets, particularly in Asia-Pacific and Latin America.
The market for rare disease treatments is highly competitive, with many pharmaceutical companies investing heavily in research and development to bring new therapies to market. In addition, there is a growing focus on developing personalized treatments for rare diseases, which take into account the unique genetic and molecular characteristics of individual patients.
Overall, the market for rare disease treatments is expected to continue to grow in the coming years as more therapies are developed and as the focus on personalized medicine and genetic treatments increases. However, there are also challenges associated with developing treatments for rare diseases, including the high cost of research and development, the small patient populations, and the difficulty in conducting clinical trials for these conditions.
Receptors as Target for Drug Discovery

Receptors are proteins found on the surface of cells or within cells that bind to specific molecules, such as hormones, neurotransmitters, or drugs. When a molecule binds to a receptor, it triggers a series of biochemical reactions within the cell that can lead to a variety of physiological effects.
Receptors are important targets for drug discovery because they play a critical role in regulating many physiological processes, such as metabolism, inflammation, and neurotransmission. By targeting specific receptors with drugs, it is possible to modulate their activity and affect the underlying physiological processes in a therapeutic way.
There are many different types of receptors, and each type has its own unique structure and function. For example, G protein-coupled receptors (GPCRs) are a large family of receptors that are involved in a wide range of physiological processes, including sensory perception, hormone signaling, and neurotransmission. Many drugs, including antihistamines, beta-blockers, and antidepressants, target GPCRs.
Ion channels are another important class of receptors that are involved in a wide range of physiological processes, including neuronal signaling, muscle contraction, and cell proliferation. Many drugs that target ion channels are used to treat diseases such as epilepsy, hypertension, and arrhythmias.
In recent years, advances in technology, such as high-throughput screening and computational modeling, have greatly accelerated the discovery of new drugs that target receptors. These approaches allow researchers to screen large numbers of compounds for their ability to bind to specific receptors and identify potential drug candidates.
Overall, targeting receptors is an important approach for drug discovery and has led to the development of many important drugs that are used to treat a wide range of diseases.
Global Market:
The use of receptors as a target for drug discovery is a rapidly growing field in the pharmaceutical industry. Receptors are proteins found on the surface or within cells that bind to specific molecules, such as hormones or neurotransmitters, to initiate a cellular response.
The global market for receptor-targeted drug discovery is expected to grow significantly over the next few years. According to a report by Market Research Future, the market is expected to reach a value of USD 2.7 billion by 2023, with a CAGR of 7.8% during the forecast period (2017-2023).
Factors driving the growth of the market include the increasing prevalence of chronic diseases, such as cancer and diabetes, and the need for more effective and targeted therapies. In addition, advancements in technology, such as high-throughput screening and virtual screening, have facilitated the discovery of new drugs targeting receptors.
The market is segmented by receptor type, therapeutic area, and region. By receptor type, the market is divided into G protein-coupled receptors (GPCRs), ligand-gated ion channels (LGICs), nuclear receptors, and others. GPCRs are the most commonly targeted receptors, as they are involved in a wide range of physiological processes and are the targets of many drugs already on the market.
By therapeutic area, the market is segmented into oncology, neurology, cardiovascular, respiratory, and others. Oncology is the largest segment, accounting for the highest market share due to the high demand for targeted therapies in cancer treatment.
Geographically, North America is the largest market, followed by Europe and Asia-Pacific. The presence of major pharmaceutical companies and research institutions, as well as favorable government initiatives and funding, are driving the growth of the market in North America and Europe.
Overall, the receptor-targeted drug discovery market is expected to continue its growth trajectory in the coming years, driven by advancements in technology and an increasing need for targeted therapies in the treatment of various diseases.
Drugs Affecting the Cardiovascular System

The cardiovascular system is responsible for circulating blood throughout the body and is critical for maintaining normal physiological function. There are many drugs that target the cardiovascular system, and these drugs are used to treat a variety of cardiovascular disorders, including hypertension, heart failure, arrhythmias, and angina.
Antihypertensive drugs: These drugs are used to lower blood pressure and reduce the risk of heart attack, stroke, and other cardiovascular events. Examples of antihypertensive drugs include ACE inhibitors, beta-blockers, calcium channel blockers, and diuretics.
Antiarrhythmic drugs: These drugs are used to treat abnormal heart rhythms, or arrhythmias, which can be life-threatening. Examples of antiarrhythmic drugs include sodium channel blockers, beta-blockers, and potassium channel blockers.
Anticoagulants and antiplatelet drugs: These drugs are used to prevent blood clots and reduce the risk of stroke, heart attack, and other cardiovascular events. Examples of anticoagulants include heparin and warfarin, while examples of antiplatelet drugs include aspirin and clopidogrel.
Vasodilators: These drugs dilate blood vessels, which can help reduce blood pressure and improve blood flow to the heart. Examples of vasodilators include nitroglycerin and nitrates.
Inotropic drugs: These drugs increase the strength of heart contractions and can be used to treat heart failure. Examples of inotropic drugs include digoxin and dobutamine.
Statins: These drugs lower cholesterol levels in the blood and can reduce the risk of heart attack and stroke. Examples of statins include atorvastatin and simvastatin.
Overall, drugs that target the cardiovascular system play an important role in treating a variety of cardiovascular disorders and can help improve outcomes and quality of life for patients with these conditions.
Global Market:
The global market for drugs affecting the cardiovascular system is a significant segment of the pharmaceutical industry, given the high prevalence of cardiovascular diseases and the increasing demand for effective treatments.
According to a report by Zion Market Research, the global market for cardiovascular drugs was valued at USD 82.6 billion in 2020 and is expected to reach USD 114.4 billion by 2028, growing at a CAGR of 4.2% during the forecast period (2021-2028).
The market for drugs affecting the cardiovascular system is segmented into various categories based on drug class, including anti-hypertensives, anti-dyslipidemics, anti-thrombotics, anti-arrhythmics, and others. Anti-hypertensives, which lower blood pressure, are the largest segment of the market, followed by anti-dyslipidemics, which lower cholesterol levels.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of cardiovascular diseases in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for drugs affecting the cardiovascular system is driven by several factors, including the aging population, unhealthy lifestyles, and increasing incidence of cardiovascular diseases such as hypertension, coronary artery disease, and heart failure. Additionally, the rise in healthcare expenditure and the growing focus on preventive healthcare are also contributing to the growth of the market.
Despite the promising growth prospects, the market for drugs affecting the cardiovascular system is facing some challenges, including the high cost of research and development, stringent regulatory requirements, and the increasing preference for non-pharmacological therapies.
Overall, the market for drugs affecting the cardiovascular system is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for cardiovascular diseases and advancements in drug development technology.
Drugs Affecting the Central Nervous System

Drugs that affect the central nervous system (CNS) act on the brain and spinal cord and can be used to treat a variety of neurological and psychiatric disorders. Here are some examples of drugs that affect the CNS:
Analgesics: These drugs are used to relieve pain and include opioids, such as morphine and fentanyl, as well as non-opioid drugs, such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).
Anxiolytics and hypnotics: These drugs are used to treat anxiety and sleep disorders, respectively. Examples of anxiolytics include benzodiazepines, such as diazepam and alprazolam, while examples of hypnotics include zolpidem and eszopiclone.
Antidepressants: These drugs are used to treat depression and include selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine and sertraline, as well as tricyclic antidepressants, such as amitriptyline.
Antipsychotics: These drugs are used to treat psychotic disorders, such as schizophrenia, and include typical antipsychotics, such as chlorpromazine and haloperidol, as well as atypical antipsychotics, such as risperidone and olanzapine.
Stimulants: These drugs are used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy and include drugs such as methylphenidate and amphetamines.
Mood stabilizers: These drugs are used to treat bipolar disorder and include lithium and valproic acid.
Sedatives: These drugs are used to induce sleep or reduce anxiety and include barbiturates, such as phenobarbital, and benzodiazepines, such as lorazepam.
Psychostimulants: These drugs are used to improve cognitive function, and include drugs such as modafinil and armodafinil.
Overall, drugs that affect the CNS play an important role in the treatment of neurological and psychiatric disorders, and can help improve outcomes and quality of life for patients with these conditions.
Global Market:
The global market for drugs affecting the central nervous system (CNS) is a significant segment of the pharmaceutical industry, given the high prevalence of neurological and psychiatric disorders and the increasing demand for effective treatments.
According to a report by Zion Market Research, the global CNS drugs market was valued at USD 77.2 billion in 2020 and is expected to reach USD 112.7 billion by 2028, growing at a CAGR of 4.7% during the forecast period (2021-2028).
The market for drugs affecting the CNS is segmented into various categories based on drug class, including anti-depressants, anti-psychotics, anti-epileptics, anxiolytics, and others. Anti-depressants are the largest segment of the market, followed by anti-psychotics.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of neurological and psychiatric disorders in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for drugs affecting the CNS is driven by several factors, including the increasing prevalence of neurological and psychiatric disorders such as depression, anxiety, schizophrenia, and epilepsy. Additionally, the rise in healthcare expenditure and the growing focus on mental health are also contributing to the growth of the market.
Despite the promising growth prospects, the market for drugs affecting the CNS is facing some challenges, including the high cost of research and development, stringent regulatory requirements, and the increasing preference for non-pharmacological therapies.
Overall, the market for drugs affecting the CNS is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for neurological and psychiatric disorders and advancements in drug development technology.
Drugs Affecting Hormonal Systems

Hormones are chemicals produced by the endocrine system that regulate various physiological functions in the body. Drugs that affect hormonal systems act on these hormones to modify their actions, and can be used to treat a variety of endocrine disorders. Here are some examples of drugs that affect hormonal systems:
Insulin: Insulin is a hormone produced by the pancreas that regulates glucose metabolism in the body. Insulin therapy is used to treat diabetes, a condition in which the body does not produce or properly use insulin.
Thyroid hormone: The thyroid gland produces thyroid hormones that regulate metabolism in the body. Synthetic thyroid hormone, such as levothyroxine, is used to treat hypothyroidism, a condition in which the thyroid gland does not produce enough thyroid hormone.
Glucocorticoids: Glucocorticoids are hormones produced by the adrenal gland that regulate immune function and metabolism. Synthetic glucocorticoids, such as prednisone, are used to treat inflammation and autoimmune disorders.
Androgens and estrogens: Androgens and estrogens are sex hormones produced by the gonads that regulate secondary sex characteristics and reproductive function. Synthetic androgens, such as testosterone, are used to treat hypogonadism, a condition in which the gonads do not produce enough testosterone. Synthetic estrogens, such as estradiol, are used to treat menopausal symptoms and other hormonal disorders.
Gonadotropins: Gonadotropins are hormones produced by the pituitary gland that regulate the function of the gonads. Synthetic gonadotropins, such as human chorionic gonadotropin (hCG), are used to treat infertility and other hormonal disorders.
Overall, drugs that affect hormonal systems play an important role in the treatment of endocrine disorders and can help improve outcomes and quality of life for patients with these conditions.
Global Market:
The global market for drugs affecting hormonal systems is a significant segment of the pharmaceutical industry, given the importance of hormones in regulating many physiological processes and the prevalence of endocrine disorders.
According to a report by Grand View Research, the global market for hormonal drugs was valued at USD 26.8 billion in 2020 and is expected to reach USD 41.5 billion by 2028, growing at a CAGR of 5.3% during the forecast period (2021-2028).
The market for drugs affecting hormonal systems is segmented into various categories based on drug class, including thyroid hormones, insulin, growth hormones, and others. The largest segment of the market is thyroid hormones, followed by insulin.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of endocrine disorders in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for drugs affecting hormonal systems is driven by several factors, including the increasing prevalence of endocrine disorders such as diabetes, thyroid disorders, and growth hormone deficiencies. Additionally, the rise in healthcare expenditure and the growing focus on personalized medicine are also contributing to the growth of the market.
Despite the promising growth prospects, the market for drugs affecting hormonal systems is facing some challenges, including the high cost of research and development, regulatory hurdles, and the increasing preference for non-pharmacological therapies.
Overall, the market for drugs affecting hormonal systems is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for endocrine disorders and advancements in drug development technology.
Chemotherapeutic Agents

Chemotherapeutic agents are drugs used to treat cancer by targeting rapidly dividing cells, which include cancer cells. Here are some examples of chemotherapeutic agents:
Alkylating agents: These drugs interfere with DNA replication by adding an alkyl group to the DNA molecule, which prevents the cancer cells from dividing. Examples of alkylating agents include cyclophosphamide and cisplatin.
Antimetabolites: These drugs interfere with the metabolic processes required for DNA synthesis, preventing the cancer cells from dividing. Examples of antimetabolites include methotrexate and 5-fluorouracil.
Topoisomerase inhibitors: These drugs interfere with the enzymes that help to unwind and wind DNA during replication, causing DNA damage and preventing the cancer cells from dividing. Examples of topoisomerase inhibitors include etoposide and irinotecan.
Mitotic inhibitors: These drugs interfere with cell division by preventing the formation of the mitotic spindle, which is necessary for cell division. Examples of mitotic inhibitors include paclitaxel and vinblastine.
Hormone therapies: These drugs interfere with the hormones that are required for the growth and survival of certain types of cancer cells. Examples of hormone therapies include tamoxifen and aromatase inhibitors.
Immunotherapies: These drugs activate the immune system to target and kill cancer cells. Examples of immunotherapies include checkpoint inhibitors and CAR-T cell therapies.
Overall, chemotherapeutic agents are an important tool in the treatment of cancer, and can be used alone or in combination with other treatments, such as surgery and radiation therapy. The choice of chemotherapeutic agent(s) depends on the type and stage of cancer, as well as the patient's overall health and other factors.
Global Market:
The global market for chemotherapeutic agents is a significant segment of the pharmaceutical industry, given the widespread prevalence of cancer and the increasing demand for effective treatments.
According to a report by Zion Market Research, the global market for chemotherapy drugs was valued at USD 91.2 billion in 2020 and is expected to reach USD 164.3 billion by 2028, growing at a CAGR of 7.4% during the forecast period (2021-2028).
The market for chemotherapeutic agents is segmented into various categories based on drug class, including alkylating agents, anti-metabolites, anti-tumor antibiotics, and others. Alkylating agents are the largest segment of the market, followed by anti-tumor antibiotics.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of cancer in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for chemotherapeutic agents is driven by several factors, including the increasing prevalence of cancer, the aging population, and the growing focus on precision medicine. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
Despite the promising growth prospects, the market for chemotherapeutic agents is facing some challenges, including the high cost of research and development, the emergence of alternative therapies such as immunotherapy, and the increasing incidence of drug resistance.
Overall, the market for chemotherapeutic agents is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for cancer and advancements in drug development technology.
Antibacterial Agents

Antibacterial agents are drugs used to treat bacterial infections by killing or inhibiting the growth of bacteria. Here are some examples of antibacterial agents:
Beta-lactams: These drugs target the cell wall of bacteria, preventing the bacteria from dividing and leading to bacterial death. Examples of beta-lactams include penicillins, cephalosporins, and carbapenems.
Macrolides: These drugs target the ribosomes of bacteria, preventing them from producing proteins and leading to bacterial death. Examples of macrolides include erythromycin and azithromycin.
Fluoroquinolones: These drugs target DNA replication in bacteria, preventing bacterial growth and leading to bacterial death. Examples of fluoroquinolones include ciprofloxacin and levofloxacin.
Tetracyclines: These drugs target the ribosomes of bacteria, preventing them from producing proteins and leading to bacterial death. Examples of tetracyclines include doxycycline and minocycline.
Aminoglycosides: These drugs target the ribosomes of bacteria, preventing them from producing proteins and leading to bacterial death. Examples of aminoglycosides include gentamicin and tobramycin.
Sulfonamides: These drugs target the metabolic pathways of bacteria, inhibiting bacterial growth and leading to bacterial death. Examples of sulfonamides include sulfamethoxazole and trimethoprim.
Overall, antibacterial agents are an important tool in the treatment of bacterial infections, and can be used to treat a wide range of bacterial infections, from mild to severe. However, the overuse of antibacterial agents can lead to the development of antibiotic resistance, which is a growing problem that can make bacterial infections more difficult to treat. Therefore, it is important to use antibacterial agents only when necessary and as directed by a healthcare professional.
Global Market:
The global market for antibacterial agents is a significant segment of the pharmaceutical industry, given the high prevalence of bacterial infections and the increasing demand for effective treatments.
According to a report by Zion Market Research, the global market for antibacterial drugs was valued at USD 38.9 billion in 2020 and is expected to reach USD 51.3 billion by 2028, growing at a CAGR of 3.5% during the forecast period (2021-2028).
The market for antibacterial agents is segmented into various categories based on drug class, including beta-lactams, macrolides, fluoroquinolones, tetracyclines, and others. Beta-lactams are the largest segment of the market, followed by macrolides.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of bacterial infections in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for antibacterial agents is driven by several factors, including the increasing prevalence of bacterial infections, the emergence of drug-resistant bacteria, and the growing focus on infection prevention and control. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
Despite the promising growth prospects, the market for antibacterial agents is facing some challenges, including the high cost of research and development, regulatory hurdles, and the increasing preference for non-pharmacological therapies.
Overall, the market for antibacterial agents is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for bacterial infections and advancements in drug development technology.
Antiviral Agents

Antiviral agents are drugs used to treat viral infections by inhibiting the replication of viruses. Here are some examples of antiviral agents:
Nucleoside and nucleotide analogues: These drugs interfere with the replication of viral DNA or RNA by mimicking the structure of nucleosides or nucleotides, which are the building blocks of DNA and RNA. Examples of nucleoside and nucleotide analogues include acyclovir and tenofovir.
Protease inhibitors: These drugs interfere with the enzymes that are required for viral replication, preventing the virus from producing new viral particles. Examples of protease inhibitors include ritonavir and darunavir.
Entry inhibitors: These drugs prevent the virus from entering host cells, thus preventing viral replication. Examples of entry inhibitors include enfuvirtide and maraviroc.
Neuraminidase inhibitors: These drugs inhibit the activity of the neuraminidase enzyme, which is required for the release of new viral particles from infected cells. Examples of neuraminidase inhibitors include oseltamivir and zanamivir.
Interferons: These are proteins produced by the body in response to viral infections, and can be used as drugs to boost the immune response against viral infections. Examples of interferons include interferon-alpha and interferon-beta.
Overall, antiviral agents are an important tool in the treatment of viral infections, and can be used to treat a wide range of viral infections, from mild to severe. However, like with antibacterial agents, the overuse of antiviral agents can lead to the development of antiviral resistance, which is a growing problem that can make viral infections more difficult to treat. Therefore, it is important to use antiviral agents only when necessary and as directed by a healthcare professional.
Global Market:
The global market for antiviral agents is a significant segment of the pharmaceutical industry, given the high prevalence of viral infections and the increasing demand for effective treatments.
According to a report by Zion Market Research, the global market for antiviral drugs was valued at USD 42.7 billion in 2020 and is expected to reach USD 63.7 billion by 2028, growing at a CAGR of 4.8% during the forecast period (2021-2028).
The market for antiviral agents is segmented into various categories based on drug class, including nucleoside/nucleotide analogs, protease inhibitors, polymerase inhibitors, and others. Nucleoside/nucleotide analogs are the largest segment of the market, followed by protease inhibitors.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of viral infections in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for antiviral agents is driven by several factors, including the increasing prevalence of viral infections such as HIV, hepatitis, and influenza, the emergence of drug-resistant viruses, and the growing focus on vaccination and antiviral therapy for viral diseases. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
Despite the promising growth prospects, the market for antiviral agents is facing some challenges, including the high cost of research and development, regulatory hurdles, and the increasing preference for non-pharmacological therapies.
Overall, the market for antiviral agents is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for viral infections and advancements in drug development technology.
Anticancer Agents

Anticancer agents are drugs used to treat cancer by inhibiting the growth and division of cancer cells. Here are some examples of anticancer agents:
Chemotherapy agents: These drugs work by interfering with the DNA or RNA of cancer cells, thus preventing the cells from dividing and leading to cell death. Examples of chemotherapy agents include cyclophosphamide and cisplatin.
Targeted therapy agents: These drugs work by targeting specific proteins or pathways that are important for the growth and survival of cancer cells. Examples of targeted therapy agents include imatinib and trastuzumab.
Immunotherapy agents: These drugs work by boosting the immune system's ability to recognize and attack cancer cells. Examples of immunotherapy agents include checkpoint inhibitors and CAR-T cell therapy.
Hormonal therapy agents: These drugs work by blocking the effects of hormones that can stimulate the growth of certain types of cancer cells. Examples of hormonal therapy agents include tamoxifen and leuprolide.
Radiopharmaceuticals: These drugs contain radioactive particles that can be targeted to specific cancer cells, thus delivering a high dose of radiation directly to the cancer cells. Examples of radiopharmaceuticals include iodine-131 and lutetium-177.
Overall, anticancer agents are an important tool in the treatment of cancer, and can be used to treat a wide range of cancers, from early-stage to advanced-stage cancers. However, these agents can also cause side effects, some of which can be severe, and can also affect normal cells in addition to cancer cells. Therefore, it is important to use anticancer agents only when necessary and as directed by a healthcare professional.
Global Market:
The global market for anticancer agents is a significant segment of the pharmaceutical industry, given the high prevalence of cancer and the increasing demand for effective treatments.
According to a report by Zion Market Research, the global market for anticancer drugs was valued at USD 98.9 billion in 2020 and is expected to reach USD 193.8 billion by 2028, growing at a CAGR of 8.2% during the forecast period (2021-2028).
The market for anticancer agents is segmented into various categories based on drug class, including chemotherapy drugs, targeted therapy drugs, immunotherapy drugs, and hormonal therapy drugs. Chemotherapy drugs are the largest segment of the market, followed by targeted therapy drugs.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of cancer in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for anticancer agents is driven by several factors, including the increasing prevalence of cancer, the growing focus on early detection and treatment, and the increasing availability of effective therapies. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
Despite the promising growth prospects, the market for anticancer agents is facing some challenges, including the high cost of research and development, regulatory hurdles, and the increasing preference for non-pharmacological therapies.
Overall, the market for anticancer agents is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for cancer and advancements in drug development technology.
The Opioid Analgesics

Opioid analgesics, also known as narcotics, are a group of drugs used to treat moderate to severe pain. They work by binding to specific receptors in the brain and spinal cord, known as opioid receptors, which are involved in the transmission of pain signals.
Opioid analgesics can be classified as either natural, semi-synthetic, or synthetic opioids. Natural opioids, such as morphine and codeine, are derived from the opium poppy plant. Semi-synthetic opioids, such as oxycodone and hydrocodone, are chemically modified versions of natural opioids. Synthetic opioids, such as fentanyl and tramadol, are chemically synthesized compounds that mimic the effects of natural opioids.
Opioid analgesics are often used to treat pain associated with surgery, injury, or chronic conditions such as cancer or arthritis. They can be administered in various forms, including tablets, capsules, injections, and patches.
While opioid analgesics can be highly effective in treating pain, they can also have serious side effects and risks. Common side effects include constipation, nausea, drowsiness, and confusion. Long-term use of opioids can lead to physical dependence, tolerance, and addiction, and can increase the risk of overdose and death.
To minimize the risk of side effects and addiction, opioid analgesics should be used only as directed by a healthcare provider and for the shortest possible duration. It is also important to follow dosage instructions carefully and to avoid sharing or selling opioids with others. People with a history of substance abuse or addiction should use opioids with caution and under the guidance of a healthcare provider.
Global Market:
The global market for opioid analgesics is a significant segment of the pharmaceutical industry, given the high prevalence of chronic pain and the widespread use of opioids as painkillers.
According to a report by Grand View Research, the global market for opioid analgesics was valued at USD 27.8 billion in 2020 and is expected to reach USD 36.5 billion by 2028, growing at a CAGR of 3.5% during the forecast period (2021-2028).
The market for opioid analgesics is segmented into various categories based on drug class, including natural opioids, semi-synthetic opioids, and synthetic opioids. Semi-synthetic opioids are the largest segment of the market, followed by natural opioids.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of chronic pain in these regions and the widespread use of opioids for pain management. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for opioid analgesics is driven by several factors, including the increasing prevalence of chronic pain, the growing aging population, and the availability of effective pain management options. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
However, the market for opioid analgesics is also facing challenges such as the high risk of addiction, overdose, and abuse associated with these drugs. Moreover, the recent opioid crisis has resulted in increased regulatory scrutiny and stricter prescribing guidelines, which may impact the market growth.
Overall, the market for opioid analgesics is expected to continue growing in the coming years, driven by the increasing demand for effective pain management options and advancements in drug development technology, while taking into account the need to balance the benefits with the risks of opioid use.
Anti-Ulcer Agents

Anti-ulcer agents are a group of drugs used to treat ulcers in the stomach or small intestine. Ulcers are open sores that can develop in the lining of the digestive tract, and they can cause symptoms such as pain, nausea, and vomiting.
There are several types of anti-ulcer agents, including proton pump inhibitors (PPIs), H2 receptor antagonists, and antacids. PPIs, such as omeprazole and esomeprazole, work by blocking the production of acid in the stomach, which can help to reduce the formation of ulcers and promote healing. H2 receptor antagonists, such as ranitidine and famotidine, work by blocking the activity of histamine, which stimulates the production of acid in the stomach. Antacids, such as calcium carbonate and magnesium hydroxide, work by neutralizing acid in the stomach and providing relief from symptoms.
Other types of anti-ulcer agents include cytoprotective agents, such as sucralfate, which form a protective barrier on the lining of the stomach and help to prevent further damage. Antibiotics, such as amoxicillin and clarithromycin, may also be used in combination with other anti-ulcer agents to treat ulcers caused by bacterial infections, such as Helicobacter pylori.
While anti-ulcer agents can be effective in treating ulcers, they can also have side effects, particularly if used in high doses or for prolonged periods. Common side effects include diarrhea, constipation, and headache. Long-term use of PPIs has also been associated with an increased risk of bone fractures, kidney disease, and infections.
It is important to use anti-ulcer agents only under the guidance of a healthcare provider and to follow dosage instructions carefully to minimize the risk of side effects. People with a history of kidney disease or other medical conditions should use anti-ulcer agents with caution and under the guidance of a healthcare provider.
Global Market:
The global market for anti-ulcer agents is an important segment of the pharmaceutical industry, given the high prevalence of gastrointestinal disorders and the increasing demand for effective treatments.
According to a report by Zion Market Research, the global market for anti-ulcer drugs was valued at USD 35.6 billion in 2020 and is expected to reach USD 47.4 billion by 2028, growing at a CAGR of 3.6% during the forecast period (2021-2028).
The market for anti-ulcer agents is segmented into various categories based on drug class, including proton pump inhibitors (PPIs), histamine-2 receptor antagonists (H2RAs), and antacids. PPIs are the largest segment of the market, followed by H2RAs.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of gastrointestinal disorders in these regions and the presence of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for anti-ulcer agents is driven by several factors, including the increasing prevalence of gastrointestinal disorders, the growing aging population, and the availability of effective treatments. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
Despite the promising growth prospects, the market for anti-ulcer agents is facing challenges such as the emergence of drug-resistant strains, the side effects associated with prolonged use of these drugs, and the increasing preference for non-pharmacological therapies.
Overall, the market for anti-ulcer agents is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for gastrointestinal disorders and advancements in drug development technology, while also taking into account the need to balance the benefits with the risks of long-term drug use.
Non-Steroidal Anti Inflammatory Drugs

Non-steroidal anti-inflammatory drugs (NSAIDs) are a group of drugs commonly used to treat pain, inflammation, and fever. They work by blocking the production of certain chemicals in the body, known as prostaglandins, which are involved in inflammation, pain, and fever.
NSAIDs can be divided into two main categories: non-selective NSAIDs and selective NSAIDs. Non-selective NSAIDs, such as aspirin, ibuprofen, and naproxen, block the activity of both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) enzymes, which are involved in the production of prostaglandins. Selective NSAIDs, such as celecoxib, only block the activity of COX-2 enzymes, which are primarily involved in inflammation.
NSAIDs are commonly used to treat a variety of conditions, including arthritis, menstrual cramps, headaches, and muscle strains. They are available over-the-counter or by prescription, depending on the strength and formulation of the drug.
While NSAIDs are generally considered safe and effective, they can also have side effects, particularly if used in high doses or for prolonged periods. Common side effects include stomach upset, heartburn, and nausea. Long-term use of NSAIDs can also increase the risk of more serious side effects, such as stomach ulcers, bleeding, and kidney damage.
It is important to use NSAIDs only as directed by a healthcare provider and to follow dosage instructions carefully to minimize the risk of side effects. People with a history of stomach ulcers or bleeding, kidney disease, or heart disease should use NSAIDs with caution and under the guidance of a healthcare provider.
Global Market:
The global market for non-steroidal anti-inflammatory drugs (NSAIDs) is a significant segment of the pharmaceutical industry, given the widespread use of these drugs for the management of pain, inflammation, and fever.
According to a report by Grand View Research, the global market for NSAIDs was valued at USD 12.6 billion in 2020 and is expected to reach USD 18.2 billion by 2028, growing at a CAGR of 4.4% during the forecast period (2021-2028).
The market for NSAIDs is segmented into various categories based on drug class, including traditional NSAIDs and selective COX-2 inhibitors. Traditional NSAIDs are the largest segment of the market, followed by selective COX-2 inhibitors.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of pain and inflammation in these regions and the widespread use of NSAIDs for pain management. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for NSAIDs is driven by several factors, including the increasing prevalence of pain and inflammation, the growing aging population, and the availability of effective pain management options. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
However, the market for NSAIDs is also facing challenges such as the risk of gastrointestinal bleeding, cardiovascular events, and renal toxicity associated with these drugs. Moreover, the emergence of alternative therapies such as physiotherapy, acupuncture, and herbal medicines is also impacting the market growth.
Overall, the market for NSAIDs is expected to continue growing in the coming years, driven by the increasing demand for effective pain management options and advancements in drug development technology, while taking into account the need to balance the benefits with the risks of long-term drug use.
Steroids

Steroids are a group of organic compounds that are naturally produced in the human body, as well as in plants and animals. They play important roles in various physiological processes, including the regulation of metabolism, immune function, and reproduction.
In medicine, steroids are often used as medications to reduce inflammation and suppress the immune system. These drugs, known as corticosteroids, are commonly used to treat conditions such as asthma, allergies, and autoimmune diseases.
Corticosteroids work by binding to specific receptors in the body and inhibiting the production of inflammatory cytokines, which are proteins that contribute to the development of inflammation. By reducing inflammation, corticosteroids can help to alleviate symptoms such as pain, swelling, and redness.
However, corticosteroids can also have side effects, particularly if used in high doses or for prolonged periods. Common side effects include weight gain, mood changes, and increased risk of infections. Long-term use of corticosteroids can also lead to more serious side effects, such as osteoporosis, diabetes, and adrenal suppression.
Anabolic steroids, on the other hand, are a group of synthetic compounds that are chemically similar to the male hormone testosterone. These drugs are often used illicitly by athletes and bodybuilders to enhance muscle growth and performance. However, anabolic steroids can also have serious side effects, including liver damage, cardiovascular disease, and mood disorders.
It is important to use steroids only under the guidance of a healthcare provider and to follow dosage instructions carefully to minimize the risk of side effects.
Global Market:
The global market for steroids is an important segment of the pharmaceutical industry, given the wide range of therapeutic applications of these drugs, including inflammation, allergies, hormonal disorders, and autoimmune diseases.
According to a report by Grand View Research, the global market for steroids was valued at USD 10.8 billion in 2020 and is expected to reach USD 16.4 billion by 2028, growing at a CAGR of 5.4% during the forecast period (2021-2028).
The market for steroids is segmented into various categories based on drug class, including glucocorticoids, mineralocorticoids, and sex steroids. Glucocorticoids are the largest segment of the market, followed by sex steroids.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of hormonal disorders and autoimmune diseases in these regions and the availability of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for steroids is driven by several factors, including the increasing prevalence of hormonal disorders and autoimmune diseases, the growing aging population, and the availability of effective treatments. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
However, the market for steroids is also facing challenges such as the risk of adverse effects such as osteoporosis, cardiovascular events, and diabetes associated with prolonged use of these drugs. Moreover, the emergence of alternative therapies such as biologics and gene therapy is also impacting the market growth.
Overall, the market for steroids is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for hormonal disorders and autoimmune diseases and advancements in drug development technology, while also taking into account the need to balance the benefits with the risks of long-term drug use.
Antibiotics

Antibiotics are a group of drugs used to treat bacterial infections. These drugs work by either killing bacteria or inhibiting their growth and reproduction.
There are several classes of antibiotics, including penicillins, cephalosporins, macrolides, tetracyclines, and fluoroquinolones. Each class of drugs has a different mechanism of action and is effective against different types of bacteria.
Penicillins, for example, work by interfering with the ability of bacteria to build their cell walls, causing them to burst and die. Cephalosporins have a similar mechanism of action but are more effective against certain types of bacteria that are resistant to penicillins. Macrolides, on the other hand, work by inhibiting the ability of bacteria to synthesize proteins, preventing them from growing and reproducing.
Antibiotics can be administered in several different ways, including orally, topically, and intravenously. The choice of treatment will depend on the type and severity of the infection, as well as the individual patient's health status and preferences.
While antibiotics can be effective in treating bacterial infections, they can also have side effects, particularly if used inappropriately or for prolonged periods. Common side effects include nausea, diarrhea, and allergic reactions. More serious side effects, such as liver and kidney damage, may also occur in rare cases. It is important to use antibiotics judiciously and to follow dosage instructions carefully to minimize the risk of side effects and the development of antibiotic resistance.
Global Market:
Antibiotics are a class of drugs that are used to treat bacterial infections. The global market for antibiotics is a significant segment of the pharmaceutical industry, given the widespread use of these drugs for the management of bacterial infections.
According to a report by Grand View Research, the global market for antibiotics was valued at USD 43.6 billion in 2020 and is expected to reach USD 56.3 billion by 2028, growing at a CAGR of 3.3% during the forecast period (2021-2028).
The market for antibiotics is segmented into various categories based on drug class, including penicillins, cephalosporins, macrolides, fluoroquinolones, and others. Penicillins are the largest segment of the market, followed by macrolides.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of bacterial infections in these regions and the availability of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for antibiotics is driven by several factors, including the increasing prevalence of bacterial infections, the growing aging population, and the availability of effective treatments. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
However, the market for antibiotics is also facing challenges such as the emergence of antibiotic-resistant bacteria, which is limiting the effectiveness of existing antibiotics and driving the need for the development of new antibiotics. Moreover, the high cost of developing and producing new antibiotics and the stringent regulatory requirements for approval are also impacting the market growth.
Overall, the market for antibiotics is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for bacterial infections and advancements in drug development technology, while also taking into account the need to balance the benefits with the risks of antibiotic resistance.
Antineoplastic Agents

Antineoplastic agents are drugs used in the treatment of cancer. These drugs work by targeting and destroying cancer cells, either by directly killing the cells or by interfering with their ability to grow and divide.
There are several classes of antineoplastic agents, including alkylating agents, antimetabolites, topoisomerase inhibitors, and monoclonal antibodies. Each class of drugs has a different mechanism of action and is effective against different types of cancer.
Alkylating agents, for example, work by damaging the DNA of cancer cells, preventing them from dividing and growing. Antimetabolites, on the other hand, mimic the structure of normal cellular components, such as nucleotides, which cancer cells need to grow and divide. By interfering with the production of these components, antimetabolites prevent cancer cells from proliferating.
Topoisomerase inhibitors work by interfering with the enzymes that help cancer cells to unwind and replicate their DNA. Monoclonal antibodies, on the other hand, target specific proteins on the surface of cancer cells, either directly killing the cells or marking them for destruction by the immune system.
Antineoplastic agents can be administered in several different ways, including orally, intravenously, and topically. The choice of treatment will depend on the type and stage of cancer, as well as the individual patient's health status and preferences.
While antineoplastic agents can be effective in treating cancer, they can also have significant side effects, including nausea, vomiting, hair loss, and fatigue. More serious side effects, such as anemia, infections, and organ damage, may also occur. It is important to carefully monitor patients receiving antineoplastic agents and to provide supportive care as needed to manage side effects.
Global Market:
Antineoplastic agents, also known as chemotherapy drugs, are a class of drugs used to treat cancer. The global market for antineoplastic agents is a significant segment of the pharmaceutical industry, given the high prevalence of cancer and the need for effective treatments.
According to a report by Grand View Research, the global market for antineoplastic agents was valued at USD 86.2 billion in 2020 and is expected to reach USD 156.6 billion by 2028, growing at a CAGR of 7.6% during the forecast period (2021-2028).
The market for antineoplastic agents is segmented into various categories based on drug class, including alkylating agents, antimetabolites, cytotoxic antibiotics, hormonal agents, and targeted therapies. Targeted therapies are the largest segment of the market, followed by alkylating agents.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of cancer in these regions and the availability of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for antineoplastic agents is driven by several factors, including the increasing prevalence of cancer, the growing aging population, and the availability of effective treatments. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
However, the market for antineoplastic agents is also facing challenges such as the high cost of developing and producing new drugs, the limited effectiveness of existing treatments, and the emergence of drug resistance. Moreover, the stringent regulatory requirements for approval and the adverse effects associated with chemotherapy drugs are also impacting the market growth.
Overall, the market for antineoplastic agents is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for cancer and advancements in drug development technology, while also taking into account the need to balance the benefits with the risks of adverse effects and drug resistance.
Anthelmintics

Anthelmintics are a group of drugs used to treat infections caused by parasitic worms, including roundworms, tapeworms, and flukes. These drugs work by either killing the worms or by inhibiting their growth and reproduction.
There are several classes of anthelmintics, including benzimidazoles, nicotinic agonists, and macrocyclic lactones. Each class of drugs has a different mechanism of action and is effective against different types of worms.
Anthelmintics are commonly used in both humans and animals to treat a variety of infections. In humans, they are used to treat infections caused by intestinal worms, such as ascariasis, hookworm infection, and trichuriasis. They may also be used to treat infections caused by tissue-dwelling worms, such as filariasis.
In animals, anthelmintics are used to treat a variety of infections caused by parasites, including lungworms, liver flukes, and tapeworms. These drugs are commonly used in livestock to prevent and control parasitic infections, which can cause significant economic losses.
While anthelmintics are generally considered safe and effective, they can have side effects, particularly if used in high doses or for prolonged periods. Common side effects include nausea, vomiting, and abdominal pain. In rare cases, more serious side effects, such as liver and kidney damage, may occur. It is important to follow dosage instructions carefully and to consult a healthcare provider or veterinarian if you have any concerns about the use of anthelmintics.
Global Market:
Anthelmintics are drugs used to treat infections caused by parasitic worms, such as roundworms, tapeworms, and flukes. The global market for anthelmintics is a significant segment of the pharmaceutical industry, given the high prevalence of parasitic worm infections in humans and livestock.
According to a report by Grand View Research, the global market for anthelmintics was valued at USD 1.8 billion in 2020 and is expected to reach USD 2.6 billion by 2028, growing at a CAGR of 4.6% during the forecast period (2021-2028).
The market for anthelmintics is segmented into various categories based on drug class, including benzimidazoles, nicotinic agonists, macrocyclic lactones, and others. Macrocyclic lactones are the largest segment of the market, followed by benzimidazoles.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of parasitic worm infections in livestock and the availability of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for anthelmintics is driven by several factors, including the increasing prevalence of parasitic worm infections in humans and livestock, the growing demand for animal-derived products, and the availability of effective treatments. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
However, the market for anthelmintics is also facing challenges such as the emergence of drug-resistant parasites, which is limiting the effectiveness of existing treatments and driving the need for the development of new anthelmintics. Moreover, the high cost of developing and producing new drugs and the stringent regulatory requirements for approval are also impacting the market growth.
Overall, the market for anthelmintics is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for parasitic worm infections and advancements in drug development technology, while also taking into account the need to balance the benefits with the risks of drug resistance.
Sulphonamides

Sulfonamides, also known as sulphonamides, are a group of synthetic antimicrobial agents that have been widely used in the treatment of bacterial infections since the 1930s. These drugs work by inhibiting the growth and replication of bacteria by interfering with their ability to produce folic acid, a key nutrient required for the synthesis of DNA and RNA.
Sulfonamides are generally well-tolerated and have a broad spectrum of activity against a wide range of bacteria, including both gram-positive and gram-negative bacteria. They have been used to treat a variety of infections, including urinary tract infections, respiratory tract infections, and skin and soft tissue infections.
However, sulfonamides have become less commonly used in recent years due to the development of antibiotic resistance and the availability of newer, more effective antibiotics. They are also associated with a number of side effects, including allergic reactions, blood disorders, and kidney damage.
Despite their declining use, sulfonamides remain an important class of antibiotics and continue to be used in certain situations, such as in the treatment of infections caused by bacteria that are resistant to other antibiotics.
Global Market:
Sulfonamides, also known as sulfa drugs, are a class of antibiotics used to treat bacterial infections. The global market for sulfonamides is a significant segment of the pharmaceutical industry, given the wide range of bacterial infections that can be treated with these drugs.
According to a report by Research and Markets, the global sulfonamides market was valued at USD 3.3 billion in 2020 and is expected to reach USD 4.2 billion by 2027, growing at a CAGR of 3.4% during the forecast period (2021-2027).
The market for sulfonamides is segmented into various categories based on drug type, including sulfamethoxazole-trimethoprim, sulfadiazine, and others. Sulfamethoxazole-trimethoprim is the largest segment of the market, followed by sulfadiazine.
The market is also segmented by geography, with North America and Europe accounting for the largest market shares due to the high prevalence of bacterial infections and the availability of major pharmaceutical companies. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing awareness and access to healthcare, as well as rising disposable income.
The demand for sulfonamides is driven by several factors, including the increasing prevalence of bacterial infections, the growing awareness about the benefits of sulfonamide antibiotics, and the availability of effective treatments. Additionally, the rise in healthcare expenditure and the increasing availability of reimbursement policies are also contributing to the growth of the market.
However, the market for sulfonamides is also facing challenges such as the emergence of drug-resistant bacteria, which is limiting the effectiveness of existing treatments and driving the need for the development of new sulfonamide antibiotics. Moreover, the high cost of developing and producing new drugs and the stringent regulatory requirements for approval are also impacting the market growth.
Overall, the market for sulfonamides is expected to continue growing in the coming years, driven by the increasing demand for effective treatments for bacterial infections and advancements in drug development technology, while also taking into account the need to balance the benefits with the risks of drug resistance.
Artificial Intelligence and Machine Learning in Drug Discovery and Development

Artificial intelligence (AI) and machine learning (ML) are rapidly transforming drug discovery and development, providing researchers with powerful tools to accelerate drug discovery, improve target identification, and optimize drug development.
One of the most significant applications of AI and ML in drug discovery is the identification of new drug targets. By analyzing large datasets of genetic and molecular information, researchers can use AI algorithms to identify potential drug targets and predict the efficacy of drugs against specific targets. This approach can significantly reduce the time and cost involved in the early stages of drug discovery.
AI and ML can also be used to analyze large amounts of data from clinical trials, allowing researchers to identify patient subgroups that may respond differently to a particular treatment. This approach can help to personalize treatments and improve clinical outcomes.
Another important application of AI and ML in drug development is in drug design and optimization. AI algorithms can be used to design and optimize drug molecules to maximize their therapeutic potential while minimizing potential side effects. This approach has the potential to significantly reduce the time and cost involved in developing new drugs.
AI and ML can also be used in drug safety testing, helping to identify potential side effects or drug interactions before a drug is approved for use.
Overall, AI and ML are powerful tools that have the potential to transform drug discovery and development, accelerating the development of new treatments, and improving patient outcomes. However, it is important to note that AI and ML are still in their early stages of development, and there are still significant challenges to be addressed, such as ensuring the accuracy and reliability of AI algorithms and addressing ethical considerations surrounding the use of AI in healthcare.
Global Market:
The use of artificial intelligence (AI) and machine learning (ML) in drug discovery and development is rapidly gaining traction in the pharmaceutical industry. AI and ML are being applied to various stages of the drug discovery process, from target identification to clinical trial design and beyond. The global market for AI and ML in drug discovery and development is expected to grow significantly in the coming years.
According to a report by MarketsandMarkets, the global AI in drug discovery market is expected to reach USD 1,434 million by 2024, growing at a CAGR of 40.8% from 2019 to 2024. Similarly, the global market for ML in drug discovery and development is expected to reach USD 4,015 million by 2025, growing at a CAGR of 40.8% from 2020 to 2025, according to a report by ResearchAndMarkets.
The use of AI and ML in drug discovery and development has several benefits, including faster and more efficient drug discovery, reduced costs, and improved success rates in clinical trials. AI and ML can help to identify new drug targets, predict drug efficacy and toxicity, and optimize clinical trial design, among other applications.
The market for AI and ML in drug discovery and development is segmented by technology, application, and geography. By technology, the market is segmented into machine learning, deep learning, and natural language processing, among others. By application, the market is segmented into target identification and validation, lead identification and optimization, and clinical trial design and prediction, among others.
The market is dominated by North America, followed by Europe, due to the high adoption rate of AI and ML in drug discovery and development and the presence of major pharmaceutical companies in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing investments in the healthcare industry and growing adoption of AI and ML technologies in drug discovery and development.
The key players in the market include IBM Corporation, Microsoft Corporation, Google LLC, NVIDIA Corporation, Atomwise Inc., BenevolentAI Ltd., Exscientia Ltd., Insilico Medicine, Inc., Numerate, Inc., and Cloud Pharmaceuticals, Inc., among others.
Overall, the use of AI and ML in drug discovery and development is expected to revolutionize the pharmaceutical industry, providing faster and more efficient drug discovery, and leading to the development of more effective treatments for various diseases.
Genomics and Proteomics in Drug Discovery and Development

Genomics and proteomics have become crucial areas of study in drug discovery and development, providing valuable insights into the underlying molecular mechanisms of diseases and enabling the development of targeted therapies.
Genomics involves the study of an organism's complete set of DNA, including its genes and their functions. Advances in genomics have led to the identification of numerous disease-related genes, which has opened up new avenues for drug discovery. For example, identifying specific gene mutations in cancer cells has led to the development of targeted therapies that specifically target these mutations.
Proteomics, on the other hand, involves the study of an organism's complete set of proteins, including their structures, functions, and interactions. Proteomics has been used to identify new drug targets and to gain a deeper understanding of the underlying mechanisms of diseases.
One important application of genomics and proteomics in drug discovery is in the development of personalized medicine. By analyzing an individual's genetic and proteomic profile, researchers can identify specific molecular targets that are unique to that individual's disease and develop personalized therapies that are tailored to their specific needs.
Another key application of genomics and proteomics is in drug safety and efficacy testing. By analyzing how drugs interact with specific proteins and genes, researchers can better predict how a drug will behave in the body and identify potential side effects or drug interactions.
Overall, genomics and proteomics are essential tools in drug discovery and development, providing researchers with valuable insights into the underlying molecular mechanisms of diseases and enabling the development of targeted and personalized therapies that have the potential to transform healthcare.
Global Market:
Genomics and proteomics are rapidly becoming integral parts of drug discovery and development. Genomics is the study of an organism's entire DNA sequence, while proteomics is the study of an organism's entire set of proteins. Both fields offer valuable insights into the molecular basis of diseases and the development of targeted therapies. The global market for genomics and proteomics in drug discovery and development is expected to grow significantly in the coming years.
According to a report by Grand View Research, the global genomics market size is expected to reach USD 29.8 billion by 2025, growing at a CAGR of 9.8% from 2019 to 2025. Similarly, the global proteomics market size is expected to reach USD 52.2 billion by 2027, growing at a CAGR of 13.1% from 2020 to 2027, according to a report by Reports and Data.
The use of genomics and proteomics in drug discovery and development has several benefits, including the ability to identify new drug targets, better understand disease mechanisms, and develop more targeted and personalized therapies. Genomics and proteomics can also help to optimize clinical trial design and improve patient stratification, leading to more efficient drug development and higher success rates in clinical trials.
The market for genomics and proteomics in drug discovery and development is segmented by technology, application, and geography. By technology, the market is segmented into genomics technologies (DNA sequencing, PCR, microarrays, and others) and proteomics technologies (mass spectrometry, protein microarrays, and others). By application, the market is segmented into target identification, biomarker discovery, and clinical trials, among others.
The market is dominated by North America, followed by Europe, due to the high adoption rate of genomics and proteomics in drug discovery and development and the presence of major pharmaceutical companies in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing investments in the healthcare industry and growing adoption of genomics and proteomics technologies in drug discovery and development.
The key players in the market include Illumina, Thermo Fisher Scientific, Agilent Technologies, Bio-Rad Laboratories, Danaher Corporation, Merck KGaA, Waters Corporation, Bruker Corporation, Shimadzu Corporation, and PerkinElmer, among others.
Overall, the use of genomics and proteomics in drug discovery and development is expected to continue to grow, leading to the development of more effective and personalized therapies for a wide range of diseases.
Gene Therapy and Genome Editing in Treating Genetic Diseases

Gene therapy and genome editing are promising approaches for treating genetic diseases by modifying the underlying genetic defect. Here are some ways in which gene therapy and genome editing are being used in the treatment of genetic diseases:
Gene replacement therapy: In gene replacement therapy, a functional copy of a defective gene is introduced into the patient's cells to replace the defective gene. This approach has been used successfully to treat genetic disorders such as severe combined immunodeficiency (SCID) and spinal muscular atrophy (SMA).
Gene editing: Gene editing technologies such as CRISPR-Cas9 can be used to precisely edit the DNA sequence of a patient's cells to correct a genetic defect. This approach has shown promise in preclinical studies for treating genetic disorders such as cystic fibrosis, sickle cell anemia, and Huntington's disease.
RNA interference (RNAi): RNAi is a gene therapy approach that involves using small RNA molecules to silence the expression of a defective gene. This approach has been used to treat genetic disorders such as amyloidosis and hereditary transthyretin-mediated amyloidosis.
Gene augmentation therapy: In gene augmentation therapy, additional copies of a functional gene are introduced into the patient's cells to increase the expression of the functional gene. This approach has been used to treat genetic disorders such as hemophilia and retinal dystrophies.
Genome-wide association studies (GWAS): GWAS can be used to identify genetic variants associated with a particular disease or condition. This information can be used to develop new therapies and to personalize existing therapies based on an individual's genetic profile.
Overall, gene therapy and genome editing are promising approaches for treating genetic diseases, but more research is needed to address safety and efficacy concerns, as well as to develop scalable and cost-effective manufacturing processes for these therapies.
Global Market:
Gene therapy and genome editing are two rapidly evolving fields that offer the potential to treat genetic diseases by targeting the underlying genetic causes of the diseases. Gene therapy involves delivering a functional gene to replace or compensate for a defective or missing gene, while genome editing involves precisely modifying or correcting a patient's own genetic code to address the underlying cause of the disease.
The global market for gene therapy and genome editing in treating genetic diseases is expected to grow significantly in the coming years. According to a report by MarketsandMarkets, the global gene therapy market is expected to reach USD 13.0 billion by 2024, growing at a CAGR of 40.8% from 2019 to 2024. Similarly, the global genome editing market is expected to reach USD 11.2 billion by 2027, growing at a CAGR of 14.3% from 2020 to 2027, according to a report by Grand View Research.
The use of gene therapy and genome editing in treating genetic diseases has several benefits, including the ability to address the underlying genetic causes of the diseases and potentially provide a cure for patients. Gene therapy and genome editing can also be used to develop personalized therapies for individual patients based on their specific genetic mutations.
The market for gene therapy and genome editing in treating genetic diseases is segmented by application, technology, and geography. By application, the market is segmented into monogenic diseases, cancer, and others. By technology, the market is segmented into viral vectors, non-viral vectors, CRISPR/Cas9, and others.
The market is dominated by North America, followed by Europe, due to the high adoption rate of gene therapy and genome editing in these regions and the presence of major pharmaceutical and biotech companies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing investments in the healthcare industry and growing adoption of gene therapy and genome editing technologies in treating genetic diseases.
The key players in the market include bluebird bio, Inc., CRISPR Therapeutics AG, Editas Medicine, Inc., Intellia Therapeutics, Inc., Sangamo Therapeutics, Inc., Spark Therapeutics, Inc., Vertex Pharmaceuticals Incorporated, and others.
Overall, the use of gene therapy and genome editing in treating genetic diseases is expected to continue to grow, leading to the development of more effective and personalized therapies for a wide range of genetic diseases.
Nanotechnology in Drug Delivery and Imaging

Nanotechnology has revolutionized drug delivery and imaging in medicine by enabling the design and development of nanoscale drug delivery systems and imaging agents. These nanoscale systems can deliver drugs and imaging agents more efficiently and effectively, resulting in improved therapeutic outcomes and diagnostic accuracy. Here are some ways in which nanotechnology is being used in drug delivery and imaging:
Targeted drug delivery: Nanoparticles can be designed to target specific cells or tissues in the body, allowing for more efficient and effective drug delivery. This can reduce side effects and improve the efficacy of the drug.
Controlled drug release: Nanoparticles can be designed to release drugs at a specific time or in response to a particular stimulus, such as changes in pH or temperature. This can improve drug efficacy and reduce side effects.
Imaging agents: Nanoparticles can be used as imaging agents for a range of imaging modalities, including magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET). Nanoparticles can enhance imaging resolution and sensitivity, enabling earlier and more accurate disease detection.
Personalized medicine: Nanoparticles can be designed to deliver drugs or imaging agents specific to individual patients or patient subgroups, allowing for personalized medicine approaches that can improve therapeutic outcomes and reduce adverse effects.
Biomarker detection: Nanoparticles can be designed to detect and quantify specific biomarkers, such as tumor markers, allowing for early detection of diseases and more accurate disease diagnosis and monitoring.
Overall, nanotechnology has the potential to improve drug delivery and imaging in medicine by enabling the development of more effective and efficient drug delivery systems and imaging agents. However, there are still challenges that need to be addressed, such as the potential toxicity of nanoparticles and the need for more robust and reproducible manufacturing processes.
Global Market:
Nanotechnology has emerged as a promising tool for drug delivery and imaging due to its unique properties such as high surface area, size, and surface charge. The use of nanotechnology in drug delivery allows for the targeted delivery of drugs to specific cells and tissues, resulting in improved therapeutic efficacy and reduced toxicity. Additionally, nanotechnology-based imaging techniques allow for enhanced visualization of biological structures and processes.
The global market for nanotechnology in drug delivery and imaging is expected to grow significantly in the coming years. According to a report by MarketsandMarkets, the global market for nanotechnology in drug delivery is expected to reach USD 98.9 billion by 2025, growing at a CAGR of 14.2% from 2020 to 2025. Similarly, the global market for nanotechnology-based imaging is expected to reach USD 7.5 billion by 2025, growing at a CAGR of 9.9% from 2020 to 2025, according to a report by Grand View Research.
The market for nanotechnology in drug delivery and imaging is segmented by application, technology, and geography. By application, the market is segmented into oncology, cardiovascular diseases, neurology, anti-inflammatory/immunology, and others. By technology, the market is segmented into nanoparticle-based drug delivery, liposomes, polymeric nanoparticles, and others.
The market is dominated by North America, followed by Europe, due to the high adoption rate of nanotechnology in these regions and the presence of major pharmaceutical and biotech companies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing investments in the healthcare industry and growing adoption of nanotechnology-based drug delivery and imaging technologies.
The key players in the market include AbbVie Inc., Amgen Inc., AstraZeneca plc, Merck & Co., Inc., Novartis AG, Pfizer Inc., Roche Holding AG, and others.
Overall, the use of nanotechnology in drug delivery and imaging has the potential to revolutionize the way drugs are delivered and how diseases are diagnosed and monitored. The market is expected to continue to grow as new technologies are developed and more applications are discovered, leading to the development of more effective and personalized therapies for a wide range of diseases.
Virtual and Augmented Reality in Drug Discovery and Development

Virtual and augmented reality technologies are increasingly being used in drug discovery and development to enhance the drug development process, improve patient outcomes, and reduce costs. Here are some ways in which virtual and augmented reality are being used in this field:
Drug discovery: Virtual and augmented reality can be used to design and simulate the structure of molecules, which can help in the identification of potential drug targets and drug candidates.
Drug development: Virtual and augmented reality can be used to simulate the effects of drugs on the human body, which can help in the design and optimization of clinical trials, reducing the time and cost of drug development.
Medical education and training: Virtual and augmented reality can be used to provide medical students and healthcare professionals with immersive, interactive training on drug therapy, drug administration, and patient care.
Patient education and support: Virtual and augmented reality can be used to provide patients with educational materials and support for medication adherence, drug administration, and disease management.
Clinical trial recruitment and retention: Virtual and augmented reality can be used to improve patient recruitment and retention in clinical trials by providing patients with an immersive, engaging experience that can improve patient engagement and adherence to treatment.
Overall, virtual and augmented reality technologies have the potential to revolutionize drug discovery and development by providing drug developers, healthcare professionals, and patients with new tools for drug design, drug development, and patient care. However, more research is needed to evaluate the efficacy and safety of these technologies and to ensure that they are used responsibly and ethically.
Global Market:
Virtual and augmented reality (VR/AR) technologies are increasingly being used in drug discovery and development to enhance visualization, simulation, and modeling of biological structures and processes. These technologies can help researchers in drug discovery by enabling them to visualize complex molecular structures, simulate drug interactions, and test hypotheses in a more efficient and cost-effective manner. In drug development, VR/AR can aid in clinical trials by providing immersive and interactive environments for patients and healthcare professionals.
The global market for VR/AR in drug discovery and development is expected to grow significantly in the coming years. According to a report by Grand View Research, the global market for VR/AR in healthcare is expected to reach USD 5.1 billion by 2025, growing at a CAGR of 28.6% from 2020 to 2025. The market is driven by factors such as the increasing adoption of VR/AR technologies in medical training and education, rising demand for minimally invasive surgeries, and the growing need for effective drug discovery and development processes.
The market for VR/AR in drug discovery and development is segmented by component, technology, and application. By component, the market is segmented into hardware, software, and services. By technology, the market is segmented into VR and AR. By application, the market is segmented into drug discovery, preclinical development, and clinical trials.
North America dominates the market for VR/AR in drug discovery and development, followed by Europe, due to the high adoption rate of these technologies in these regions and the presence of major pharmaceutical and biotech companies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing investments in the healthcare industry and growing adoption of VR/AR technologies in drug discovery and development.
The key players in the market include Alphabet Inc., BioDigital, Inc., EON Reality Inc., Nanome Inc., Osso VR Inc., Pharmaceutical Product Development LLC, SimX Inc., and WorldViz LLC, among others.
Overall, the use of VR/AR technologies in drug discovery and development has the potential to revolutionize the way drugs are discovered and developed, leading to more efficient and effective processes and ultimately better patient outcomes. The market is expected to continue to grow as new technologies and applications are developed, leading to more widespread adoption of these technologies in the pharmaceutical and biotech industries.
Patient Engagement and Patient-Centered Drug Development

Patient engagement and patient-centered drug development are approaches that prioritize the involvement of patients throughout the drug development process to ensure that their perspectives and needs are taken into account. By involving patients in drug development, drug developers can create more effective and relevant drugs that are better aligned with patient needs and preferences.
Here are some ways in which patient engagement and patient-centered drug development can be implemented:
Patient input in drug development: Patients can be involved in the early stages of drug development to provide input on the design of clinical trials, selection of endpoints, and selection of outcomes that are meaningful to patients.
Patient-reported outcomes (PROs): PROs are assessments of the patient's health status that are reported directly by the patient. By using PROs, drug developers can better understand the patient experience of a particular disease and the impact of treatment on patient outcomes.
Patient advocacy groups: Patient advocacy groups can provide valuable insights into the needs and preferences of patients and can help to mobilize patient participation in clinical trials.
Collaborative decision-making: In patient-centered drug development, patients are involved in collaborative decision-making with drug developers, regulators, and healthcare providers to ensure that their perspectives are considered throughout the drug development process.
Patient education and support: Drug developers can provide patients with information about the drug development process, clinical trials, and treatment options to help them make informed decisions about their care.
Overall, patient engagement and patient-centered drug development are critical to improving the relevance and effectiveness of drugs and ensuring that patients are at the center of the drug development process. By engaging patients as partners in drug development, drug developers can create more effective and relevant drugs that better meet the needs and preferences of patients.
Global Market:
Patient engagement and patient-centered drug development are becoming increasingly important in the pharmaceutical industry as patients play a more active role in the drug development process. Patient engagement involves actively involving patients in the drug development process, including design, development, and clinical trials, to ensure that their needs and preferences are considered. Patient-centered drug development aims to prioritize patient needs and preferences in the development of new drugs.
The global market for patient engagement and patient-centered drug development is expected to grow significantly in the coming years. According to a report by MarketsandMarkets, the global patient engagement solutions market is expected to reach USD 19.3 billion by 2025, growing at a CAGR of 16.2% from 2020 to 2025. The market is driven by factors such as the increasing focus on patient-centered care, the growing use of mobile health (mHealth) technologies, and the increasing adoption of electronic health records (EHRs) by healthcare providers.
The market for patient engagement and patient-centered drug development is segmented by component, delivery mode, application, and end user. By component, the market is segmented into software, services, and hardware. By delivery mode, the market is segmented into web-based, cloud-based, and on-premise. By application, the market is segmented into health management, home healthcare management, social and behavioral management, and financial health management. By end user, the market is segmented into healthcare providers, payers, patients, and others.
North America dominates the market for patient engagement and patient-centered drug development, followed by Europe, due to the high adoption rate of patient-centered care in these regions and the presence of major pharmaceutical and biotech companies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing investments in the healthcare industry and growing adoption of patient engagement technologies in drug development.
The key players in the market include Allscripts Healthcare Solutions, Inc., Athenahealth, Inc., Cerner Corporation, IBM Corporation, McKesson Corporation, Medecision Inc., Orion Health Ltd., and Optum, Inc., among others.
Overall, the focus on patient engagement and patient-centered drug development is expected to continue to grow as patients become more involved in the drug development process and as the pharmaceutical industry seeks to develop more effective and personalized treatments. The market for patient engagement and patient-centered drug development is expected to continue to grow as new technologies and applications are developed, leading to more widespread adoption of these technologies in the pharmaceutical and biotech industries.
Big Data Analytics in Drug Discovery and Development

Big data analytics refers to the process of analyzing large and complex data sets using advanced computing technologies and statistical algorithms to uncover patterns, correlations, and insights that can inform decision-making. In the field of drug discovery and development, big data analytics can be used to accelerate the discovery of new drugs, optimize the development process, and improve patient outcomes.
Here are some ways in which big data analytics is being used in drug discovery and development:
Data mining: Large-scale databases of genetic and clinical information can be used to identify potential drug targets and biomarkers for disease diagnosis and prognosis.
Predictive modeling: Machine learning algorithms can be used to predict the efficacy and safety of drug candidates, reducing the time and cost of drug development.
Real-world data analysis: Electronic health records, claims data, and social media data can be analyzed to identify drug safety signals and optimize patient selection for clinical trials.
High-throughput screening: Large-scale screening platforms can rapidly screen thousands of compounds for drug activity, allowing for the identification of new drug candidates.
Personalized medicine: The use of genomic and other data to personalize drug therapy for individual patients can improve drug efficacy and reduce the risk of adverse events.
Overall, big data analytics is transforming the field of drug discovery and development by enabling faster, more efficient, and more personalized approaches to drug development. However, the use of big data also raises ethical and privacy concerns that must be addressed to ensure the responsible use of this technology.
Global Market:
Patient engagement and patient-centered drug development are becoming increasingly important in the pharmaceutical industry as patients play a more active role in the drug development process. Patient engagement involves actively involving patients in the drug development process, including design, development, and clinical trials, to ensure that their needs and preferences are considered. Patient-centered drug development aims to prioritize patient needs and preferences in the development of new drugs.
The global market for patient engagement and patient-centered drug development is expected to grow significantly in the coming years. According to a report by MarketsandMarkets, the global patient engagement solutions market is expected to reach USD 19.3 billion by 2025, growing at a CAGR of 16.2% from 2020 to 2025. The market is driven by factors such as the increasing focus on patient-centered care, the growing use of mobile health (mHealth) technologies, and the increasing adoption of electronic health records (EHRs) by healthcare providers.
The market for patient engagement and patient-centered drug development is segmented by component, delivery mode, application, and end user. By component, the market is segmented into software, services, and hardware. By delivery mode, the market is segmented into web-based, cloud-based, and on-premise. By application, the market is segmented into health management, home healthcare management, social and behavioral management, and financial health management. By end user, the market is segmented into healthcare providers, payers, patients, and others.
North America dominates the market for patient engagement and patient-centered drug development, followed by Europe, due to the high adoption rate of patient-centered care in these regions and the presence of major pharmaceutical and biotech companies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing investments in the healthcare industry and growing adoption of patient engagement technologies in drug development.
The key players in the market include Allscripts Healthcare Solutions, Inc., Athenahealth, Inc., Cerner Corporation, IBM Corporation, McKesson Corporation, Medecision Inc., Orion Health Ltd., and Optum, Inc., among others.
Overall, the focus on patient engagement and patient-centered drug development is expected to continue to grow as patients become more involved in the drug development process and as the pharmaceutical industry seeks to develop more effective and personalized treatments. The market for patient engagement and patient-centered drug development is expected to continue to grow as new technologies and applications are developed, leading to more widespread adoption of these technologies in the pharmaceutical and biotech industries.
Market Value of Medicinal Chemistry in USA
The medicinal chemistry market in the USA is a growing industry that plays a critical role in drug discovery and development. The USA is home to many of the world's leading pharmaceutical companies and research institutions, which are driving innovation and pushing the boundaries of drug discovery and development.
According to a report by Grand View Research, the global medicinal chemistry market was valued at USD 281.9 billion in 2020 and is expected to grow at a CAGR of 5.6% from 2021 to 2028. This growth is driven by the increasing prevalence of chronic diseases, the growing demand for personalized medicine, and the increasing adoption of advanced technologies such as AI and genomics in drug discovery and development.
In the USA, the market is dominated by large pharmaceutical companies such as Pfizer, Merck, and Johnson & Johnson, which invest heavily in research and development to bring new drugs to market. The industry is also supported by a vibrant ecosystem of small and mid-sized biotech companies, as well as academic research institutions, that are driving innovation in the field.
The COVID-19 pandemic has also had a significant impact on the medicinal chemistry market in the USA, with many companies shifting their focus to developing treatments and vaccines for the virus. The pandemic has also highlighted the importance of drug discovery and development, and has accelerated the adoption of new technologies and approaches in the field.
Overall, the medicinal chemistry market in the USA is expected to continue to grow in the coming years, driven by advances in technology, increasing demand for personalized medicine, and the growing prevalence of chronic diseases.
Market Value of Medicinal Chemistry in Europe
Medicinal chemistry is a field that combines knowledge of chemistry, biochemistry, and pharmacology to design and develop new drugs for the treatment of diseases. It is a critical component of the pharmaceutical industry and has a significant impact on the healthcare sector.
The European pharmaceutical industry is one of the largest in the world, with many major pharmaceutical companies headquartered in Europe. According to a report by the European Federation of Pharmaceutical Industries and Associations (EFPIA), the pharmaceutical industry in Europe spent over €35 billion on research and development in 2020.
The market value of medicinal chemistry in Europe is influenced by various factors, including the demand for new drugs, the cost of research and development, regulatory requirements, and competition from generic drugs. The market is also impacted by trends in healthcare, such as the increasing prevalence of chronic diseases and the growing focus on personalized medicine.
Overall, the market value of medicinal chemistry in Europe is significant, and the field is likely to continue to play a critical role in the healthcare sector in the coming years.
Market Value of Medicinal Chemistry in Asia
The medicinal chemistry market in Asia is growing rapidly and is expected to continue to do so in the coming years. Asia is home to some of the largest pharmaceutical markets in the world, such as China, Japan, and India. The increasing demand for drugs to treat various diseases, including cancer, diabetes, and cardiovascular diseases, is driving the growth of the medicinal chemistry market in Asia.
According to a report by Mordor Intelligence, the Asian medicinal chemistry market was valued at USD 34.05 billion in 2020 and is expected to reach USD 58.58 billion by 2026, growing at a CAGR of 9.23% during the forecast period (2021-2026).
Factors contributing to the growth of the medicinal chemistry market in Asia include the increasing investment in research and development, the availability of a skilled workforce, and the growing demand for affordable and effective drugs. The increasing adoption of advanced technologies such as artificial intelligence and machine learning in drug discovery is also expected to boost the market's growth.
In summary, the medicinal chemistry market in Asia is growing rapidly and is expected to continue to do so in the coming years, driven by factors such as increasing demand for drugs, investment in research and development, and adoption of advanced technologies.
Global Market Value of Pharmaceutical Chemistry:
The pharmaceutical chemistry market is an essential component of the pharmaceutical industry, which involves the discovery, development, and manufacturing of drugs for the treatment of diseases. The market is driven by several factors, including the increasing prevalence of chronic diseases, the aging population, and the rising demand for personalized medicine.
According to a report by Zion Market Research, the global pharmaceutical chemistry market was valued at USD 44.5 billion in 2020 and is expected to reach USD 61.2 billion by 2028, growing at a CAGR of 4.1% during the forecast period (2021-2028).
The pharmaceutical chemistry market is segmented into various categories, including API synthesis, drug formulation, analytical services, and others. The API synthesis segment dominates the market, accounting for the largest share due to the increasing demand for active pharmaceutical ingredients (APIs) in drug development.
The market is also driven by increasing investment in research and development, the adoption of advanced technologies, and the growing demand for generic drugs. The pharmaceutical industry is heavily regulated, and various government bodies and organizations set the standards for drug development and manufacturing, which also influences the market's growth.
In summary, the global pharmaceutical chemistry market is growing and is expected to continue to do so in the coming years, driven by various factors such as increasing demand for APIs, investment in research and development, adoption of advanced technologies, and government regulations.
List of Medicinal Chemistry Companies:
Pfizer Inc. | AstraZeneca | Merck & Co., Inc. | Novartis AG | GlaxoSmithKline (GSK) | Johnson & Johnson | Eli Lilly and Company | Bristol-Myers Squibb | Sanofi S.A. | AbbVie Inc. | Roche Holding AG | Boehringer Ingelheim | Takeda Pharmaceutical Company Limited | Bayer AG | Astellas Pharma Inc. | Daiichi Sankyo Company Limited | Amgen Inc. | Vertex Pharmaceuticals Incorporated | Eisai Co., Ltd. | Biogen Inc. | Gilead Sciences, Inc. | Kyowa Kirin Co., Ltd. | Sumitomo Dainippon Pharma Co., Ltd. | Teva Pharmaceutical Industries Ltd. | Otsuka Pharmaceutical Co., Ltd. | Lundbeck A/S | H. Lundbeck A/S | Mallinckrodt Pharmaceuticals | Endo Pharmaceuticals Inc. | Mallinckrodt plc | Jazz Pharmaceuticals | Kyorin Pharmaceutical Co., Ltd. | The Medicines Company | Zhejiang Hisun Pharmaceutical Co., Ltd. | Alkermes plc | Sun Pharmaceutical Industries Ltd. | Taro Pharmaceutical Industries Ltd. | Zydus Cadila | Aspen Pharmacare Holdings Limited | Lupin Limited | Jubilant Life Sciences Limited | Cipla Limited | Dr. Reddy's Laboratories Ltd. | Torrent Pharmaceuticals Ltd. | Alembic Pharmaceuticals Limited | Hikal Limited | Cambrex Corporation | ChemDiv, Inc. | Hetero Drugs Limited | Torrent Pharma Inc. | Eurofins Scientific SE | Piramal Pharma Solutions | Wuxi AppTec Co., Ltd. | Jubilant Chemsys Limited | WuXi STA | Syngene International Limited | GVK BIO | Evotec SE | Charles River Laboratories International, Inc. | Lonza Group AG | Samsung BioLogics | Catalent, Inc. | Siegfried Holding AG | Cambrex Corporation | Patheon N.V. | AMRI | Fujifilm Diosynth Biotechnologies | Almac Group | Recipharm AB | Sandoz International GmbH | Laurus Labs Limited | Glenmark Pharmaceuticals Limited | Cadila Healthcare Limited | LEO Pharma A/S | Alembic Pharmaceuticals Limited | Macleods Pharmaceuticals Limited | Rusan Pharma Limited | Granules India Limited | Ind-Swift Laboratories Ltd. | Neuland Laboratories Limited | Dishman Carbogen Amcis Limited | SRS Pharmaceuticals Pvt. Ltd. | ZCL Chemicals Ltd. | USV Private Limited | Samarth Life Sciences Pvt. Ltd. | Chiral Technologies, Inc. | Chemo S.A. | Vifor Pharma Group | Alphapharm Pty Ltd | Glenmark Generics Ltd. | Biocad | Nanjing Chuanbai Pharmaceutical Co., Ltd. | Eisai Co., Ltd. | Chugai Pharmaceutical Co., Ltd. | Otsuka Pharmaceutical Co.,
List of Medicinal Chemistry Universities:
University of California, San Francisco (UCSF), USA | University of Cambridge, UK | Harvard University, USA | University of Oxford, UK | ETH Zurich, Switzerland | University of Tokyo, Japan | Massachusetts Institute of Technology (MIT), USA | University of Toronto, Canada | University of California, San Diego (UCSD), USA | Imperial College London, UK | University of Michigan, USA | Swiss Federal Institute of Technology Lausanne (EPFL), Switzerland | University of Illinois at Urbana-Champaign, USA | National University of Singapore (NUS), Singapore | University of Basel, Switzerland | University of Chicago, USA | University of California, Los Angeles (UCLA), USA | University of Pennsylvania, USA | Kyoto University, Japan | University of California, Berkeley, USA | Peking University, China | University of Texas at Austin, USA | University of California, Irvine (UCI), USA | Columbia University, USA | University of Copenhagen, Denmark | University of Wisconsin-Madison, USA | University of Manchester, UK | University of Sydney, Australia | University of California, Davis (UCD), USA | Johns Hopkins University, USA | University of Edinburgh, UK | University of Zurich, Switzerland | University of Hong Kong (HKU), Hong Kong | University of Minnesota, USA | National Taiwan University (NTU), Taiwan | University of British Columbia, Canada | University of Melbourne, Australia | Duke University, USA | University of Amsterdam, Netherlands | University of North Carolina at Chapel Hill, USA | University of Nottingham, UK | University of Heidelberg, Germany | Australian National University (ANU), Australia | Tsinghua University, China | University of Bristol, UK | University of California, Riverside (UCR), USA | University of Leeds, UK | University of Queensland, Australia | University of Alberta, Canada | University of Sheffield, UK | University of Western Australia, Australia | University of Iowa, USA | University of Glasgow, UK | University of California, Santa Cruz (UCSC), USA | University of Southampton, UK | University of Georgia, USA | University of Southern California (USC), USA | University of Vienna, Austria | University of Utah, USA | University of Warwick, UK | University of Arizona, USA | University of Paris-Sud, France | University of Virginia, USA | University of Helsinki, Finland | University of California, Santa Barbara (UCSB), USA | University of Geneva, Switzerland | University of California, Merced (UCM), USA | University of Hamburg, Germany | University of Kentucky, USA | University of Freiburg, Germany | University of Maryland, USA | University of Erlangen-Nuremberg, Germany | University of Illinois at Chicago (UIC), USA | University of Auckland, New Zealand | University of Arizona, USA | University of Oregon, USA | University of Alberta, Canada | University of Western Ontario, Canada | University of Colorado Boulder, USA | University of Bergen, Norway
List of Medicinal Chemistry Associations:
American Chemical Society, Division of Medicinal Chemistry | European Federation of Medicinal Chemistry | International Society of Heterocyclic Chemistry | International Society for the Study of Xenobiotics | American Association of Pharmaceutical Scientists | American Society of Pharmacognosy | Society for Medicinal Plant and Natural Product Research | International Society for the History of Pharmacy | International Association for Pharmaceutical Technology | International Society for Antiviral Research | International Society of Chemical Biology | International Society for Molecular Recognition | International Society for Neurochemistry | International Society for the Study of Pain | International Society for Vaccines | International Union of Basic and Clinical Pharmacology | Society of Toxicology | American Society for Mass Spectrometry | American Society for Pharmacology and Experimental Therapeutics | Royal Society of Chemistry, Biological and Medicinal Chemistry Sector | European Association of Nuclear Medicine | European Federation for Pharmaceutical Sciences | European Peptide Society | Federation of Asian Chemical Societies | International Association of Therapeutic Drug | Monitoring and Clinical Toxicology | International Chemical Biology Society | International Chemical Congress of Pacific Basin Societies | International Medicinal Chemistry Symposium | International Symposium on Reactive Intermediates and Unusual Molecules | International Society of Chemotherapy for Infection and Cancer | International Society of Nucleosides, Nucleotides and Nucleic Acids | American Society for Clinical Pharmacology and Therapeutics | Society for Laboratory Automation and Screening | Society for Medicinal Chemistry and Chemical Biology of Eastern Africa | Society of Nuclear Medicine and Molecular Imaging | European Crystallographic Association | Federation of Analytical Chemistry and Spectroscopy Societies | International Association of Pharmaceutical Scientists and Engineers | International Chemical Congress of Pacific Basin Societies | International Isotope Society | International Society for Extracellular Vesicles | International Society for the Advancement of Supercritical Fluids | International Society for the Study of Xenobiotics | Medicinal Chemistry Section of the Société Chimique de France | National Association of Industrial and Technical Chemistry | Society for Applied Spectroscopy | Society for Biological Engineering | Society for Biomaterials | Society for Chemical Hazard Communication | Society for Chemical Industry | Society for Medicinal Chemistry of Canada | Society for Pharmaceutical Dissolution Science | Society for Pharmaceutical Engineering | Society for the Study of Inborn Errors of Metabolism | Society of Chemical Manufacturers and Affiliates | Society of Cosmetic Chemists | Society of Environmental Toxicology and Chemistry | Society of Forensic Toxicologists | Society of Toxicologic Pathology | American Society for Biochemistry and Molecular Biology | American Society for Clinical Laboratory Science | American Society for Microbiology | American Society of Gene and Cell Therapy | Association of Biomolecular Resource Facilities | Association of Clinical Research Professionals | Biophysical Society | Chemical Abstracts Service | Chemical Society of Japan | Drug Information Association | European Society for Clinical Investigation | European Society for Evolutionary Biology | European Society for Gene and Cell Therapy | European Society for Paediatric Infectious Diseases | European Society for Photobiology | European Society for Virology | Federation of European Biochemical Societies
Event Venue: Corendon Amsterdam Schiphol Airport Hotel
Schipholweg 275, 1171 PK Badhoevedorp
Accommodation Venue: Corendon Amsterdam New West Hotel
Aletta Jacobslaan 7, 1066 BP Amsterdam
Amsterdam, the capital city of the Netherlands, is a vibrant and picturesque destination that attracts millions of tourists from around the world. Known for its beautiful canals, historic architecture, cultural landmarks, and liberal atmosphere, Amsterdam offers a unique blend of old-world charm and modern delights. Here are some of its top tourist destinations:
Dam Square: Located in the heart of the city, Dam Square is Amsterdam's central square and a popular gathering spot. It is home to the Royal Palace, a magnificent building that was once used as a city hall. The square is also surrounded by numerous shops, restaurants, and cafes.
Anne Frank House: A must-visit for history enthusiasts, the Anne Frank House is the actual house where Anne Frank and her family hid during World War II. It has been converted into a museum, providing visitors with a moving and educational experience about the life of Anne Frank and the Holocaust.
Van Gogh Museum: Dedicated to the works of the renowned Dutch painter Vincent van Gogh, the Van Gogh Museum is a treasure trove of his masterpieces. It houses the world's largest collection of Van Gogh's paintings, along with letters and personal artifacts that offer insight into his life and artistic journey.
Rijksmuseum: One of the most famous museums in the world, the Rijksmuseum showcases an extensive collection of Dutch art and history. It features works by Dutch masters such as Rembrandt, Vermeer, and Frans Hals. The museum's grand building is an architectural masterpiece itself.
Vondelpark: A sprawling green oasis in the heart of Amsterdam, Vondelpark is a popular recreational area for both locals and tourists. It offers picturesque walking paths, ponds, open-air theaters, and cozy cafes, making it an ideal place to relax and soak up the city's atmosphere.
Jordaan: This charming neighborhood is known for its narrow streets, picturesque canals, and historic buildings. It is a delight to explore with its boutique shops, art galleries, cozy cafes, and local markets. Jordaan offers a glimpse into the traditional Dutch way of life.
Canal Belt: Amsterdam's iconic canal belt, consisting of concentric rings of canals, is a UNESCO World Heritage site. Taking a canal boat tour is a popular way to experience the city's unique architecture, charming bridges, and houseboats. The canal belt truly embodies Amsterdam's enchanting character.
Red Light District: While controversial, the Red Light District is undoubtedly one of Amsterdam's most famous areas. Known for its legalized prostitution and vibrant nightlife, the district is a hub of entertainment, offering clubs, bars, restaurants, and adult-oriented attractions.
These are just a few highlights of Amsterdam's tourist destinations. The city is also known for its bike-friendly culture, world-class art galleries, diverse culinary scene, and colorful tulip markets. Whether you're interested in history, art, or simply enjoying the laid-back atmosphere, Amsterdam has something to offer every visitor.
Medicinal Chemists & Biochemists 22%
Pharmaceutical Scientists 18%
Drug Developers 10%
Drug Discovery Researchers 10%
Structural Biologists & Toxicologists 10%
Universities and Hospitals 10%
Pharma & Biotech Industries 15%
Postdoctoral Researchers & Students 10%


“Stay updated with the latest insights, highlights, and key takeaways from global conferences and business meetings.”
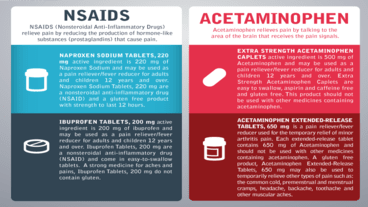
While pregnant women often self-medicate with acetaminophen (paracetamol/APAP) or NSAIDs such as ibuprofe...
View More
(E)-(R)-4-Thujanol present in thyme essential oil, is a flavoring agent with a menthol flavor. As (E)-(R)...
View More
Rare earth elements (REEs) are indispensable components in a number of technological devices, with steadi...
View More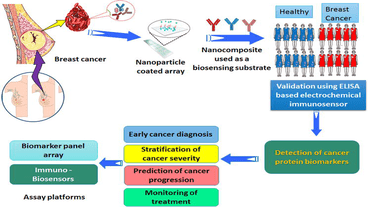
Breast cancer (BC) is one of the leading causes of death in Canadian women, with an average survival rate...
View More
Pneumonia, always a major malady, became the main public health and economic disaster of historical propo...
View More
The chest X-ray images provide vital information about the congestion cost-effectively. We propose a nove...
View More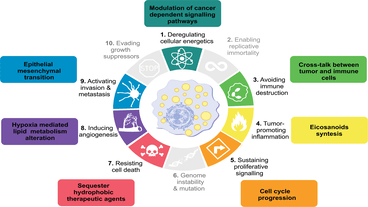
Several studies linked the bad prognostic of AML to the ability of the leukemic cells to reprogram their ...
View More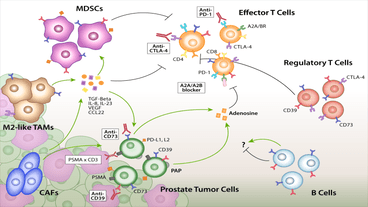
The treatment of low-risk primary prostate cancer entails active surveillance only, while high-risk disea...
View More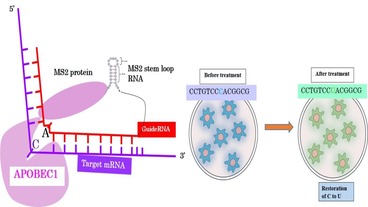
Gene therapy to supplement functional genes instead of unfunctional genes has been tried as a treatment f...
View More